Abstract
Salmonella infections in humans can range from self-limiting gastroenteritis typically associated with non-typhoidal Salmonella (NTS) to typhoidal fever, which can be life-threatening. Salmonellosis causes considerable morbidity and mortality in both humans and animals, and has a significant socioeconomic impact worldwide. In Africa, it is difficult to evaluate the situation of salmonellosis due to the non-availability of facilities capable of performing the tests essential for the diagnosis of typhoidal and non-typhoidal Salmonella infections. This article reviews important work in the literature, including the epidemiology, disease burden, pathogenesis, genomics, diagnosis, treatment, emergence and tracking of multidrug-resistant (MDR) Salmonella infections and intercontinental transmission of Salmonella to Africa. Searches of PubMed and Google Scholar were completed and the retrieved list of relevant publications were further screened. The literature revealed that the most common form of the disease in Africa is gastroenteritis, with bacterial multiplication in intestinal submucosa and diarrhoea caused by the inflammatory response and, perhaps, also by toxins. In addition to the high burden of Salmonella infection in Africa, MDR Salmonella species is on the rise in the continent, which might pose difficulties in the treatment of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella are members of the Enterobacteriaceae, facultatively anaerobic Gram-negative bacilli able to grow on a wide range of relatively simple media and distinguished from other members of the family by their biochemical characteristics and antigenic structure [1]. The genus Salmonella consists of only two major species: Salmonella enterica and Salmonella bongori. Salmonella enterica is divided into six subspecies, which are distinguished by certain biochemical characteristics and susceptibility to lysis by bacteriophage Felix 01 [2]. Salmonella enterica is divided into seven phylogenetic groups, subspecies I, II, IIIa, IIIb, IV and VII. Subspecies I includes 1367 serovars, some of which are commonly isolated from infected birds and mammals, including humans. The other subspecies mainly colonise cold-blooded vertebrates [3]. According to Su and Chiu [4], in 2005, all Salmonella species became officially recognised as a single species, S. enterica, based on their close relationship by DNA hybridisation studies. In the past, Salmonella had been named based on the original places of isolation, such as Salmonella london and Salmonella indiana. This system was replaced by the classification based on the susceptibility of isolates to different selected bacteriophages, which is also known as phage typing [5]. Phage typing has proved extremely useful for discriminating within strains of Salmonella typhimurium, virchow, enteritidis and typhi [1]. Salmonella serotypes can, however, be broadly grouped into typhoidal and non-typhoidal Salmonella, depending on the clinical syndrome associated with them [6].
Salmonella infections are of public health significance worldwide, particularly with regards to developing countries, where they are a leading cause of morbidity and mortality [7]. They cause a variety of infectious diseases in both humans and animals. The most common of such disease is gastroenteritis, with bacterial multiplication in intestinal submucosa and diarrhoea caused by the inflammatory response and, perhaps, also by toxins [3]. Infections caused by Salmonella have been categorised into four clinical types: gastroenteritis, bacteraemia or septicaemia, enteric fever and convalescent lifetime carrier state.
Kauffman–White classification
The Kauffman–White classification scheme is a system employed in the classification of Salmonella into serotypes taking cognizance of their surface antigens, the O (somatic) and H (flagella) antigens. The somatic antigens are the side chains of repeating sugar units projecting outwards from the lipopolysaccharide layer on the surface of the bacterial cell wall, while the flagella antigens are formed from structural proteins which make up the flagella. Salmonella exhibits two distinct H antigens: phases 1 and 2. An example of an antigenic formula for the serovar S. typhimurium according to the Kauffman–White scheme is 1,4,5, 12:i:1,2, where 1, 4, 5 and 12 are O antigen, i is phase 1 H antigen and 1 and 2 are phase 2 H antigen [8].
Non-typhoidal Salmonella: prevalence in Africa
Non-typhoidal Salmonella (NTS) has a worldwide occurrence. In developed countries, it is associated with mild gastrointestinal illness, which is usually self-limiting and antimicrobial treatment is not recommended [9]. In Africa, NTS infections appear to be endemic, being one of the major causes of bacteraemia, mostly in children, with 4100 deaths per year [10]. This is more prevalent in areas where malaria, malnutrition and HIV are high. Mandomando et al. [11, 12] reported a high burden of invasive NTS (S. typhimurium and S. enteritidis) infection among young children in Mozambique. Feasey et al. [13] also reported 32 % compared with 54 % of invasive NTS in children <15 years of age in South Africa and Malawi, respectively. In a similar study, Bahwere et al. [14] reported Salmonella spp. as a cause of 73 % of cases of bacteraemia in children in rural Central Africa. In Mali, three serovars S. typhimurium, S. enteritidis and S. dublin, accounted for the majority of NTS isolated from infants and young children [15]. In Côte d’Ivoire, the mortality rate was estimated to reach 5 % from Salmonella infections. From studies carried out between 2005 and 2009 among isolated serotypes, NTS were prevalent. Typing was possible for 76.1 % of strains, of which 37 % was S. typhimurium and 16 % was S. enteritidis [16]. Mohamed and Suelam [17] identified a high incidence rate (13.3 %) of Salmonella infections in human diarrhoeic cases examined in Egypt. It was found out that all serotypes (n = 4) isolated from human stool samples were S. enteritidis. NTS isolates from paediatric cases in Kenya revealed a majority to be S. typhimurium (106 out of 193, 54.9 %) and S. enteritidis (64, 33.2 %) [9]. Labi et al. [18] reported a 6.5 % (n = 181/2768) prevalence of Salmonella bacteraemia at the Korle-Bu Teaching Hospital in Ghana, with a preponderance of NTS over typhoidal Salmonella. Children under 5 years old bore the brunt of the disease. Though there has been a high prevalence of NTS in Africa, infection in some cases might be self-limiting in relatively healthy individuals. This was attributed to the infection subsiding on its own and also the possibility of intake of self-prescribed antibiotics. In a related study by Ifeanyi et al. [19], only NTS were isolated from children under 5 years old presenting with gastroenteritis in Abuja, Nigeria. The isolates were Salmonella zanzibar, Salmonella brancaster and S. enteritidis.
Another report from Nigeria by Abdullahi et al. [20] showed that, of a total of 108 Salmonella isolates from both blood and stool samples, 43.5 % were NTS while 56.5 % were typhoidal salmonellosis amongst both adults and children.
Clinical manifestation of NTS
NTS usually causes self-limiting gastroenteritis associated with nausea, abdominal pain, vomiting and inflammatory diarrhoea. In some cases, specific strains among the serovars can cause bacteraemia majorly in young children and immunocompromised patients. Incubation of NTS after ingestion of the pathogen is between 6 and 12 h [21, 22].
Emergence of antibiotic-resistant non-typhoidal Salmonella in Africa
Antibiotic-resistant NTS is becoming a major public health concern in Africa, as data from studies indicate an increase in the emergence of antibiotic-resistant strains. There have been reports of multidrug-resistant (MDR) S. typhimurium and S. enteritidis in several African countries. Mandomando et al. [11] reported a high occurrence of S. typhimurium and S. enteritidis resistant to ampicillin, chloramphenicol and trimethoprim–sulphamethoxazole in Mozambique. This is similar to the report of Oundo et al. [23], whose work revealed that 35 % of NTS isolates were resistant to ampicillin, 18 % were resistant to chloramphenicol and 39 % were resistant to sulphamethoxazole–trimethoprim in Kenya. Mengistu et al. [24] reported that more than 37 % of Salmonella serovars were resistant to ampicillin, tetracycline and co-trimoxazole in Ethiopia. In Nigeria, Abdullahi et al. [20] reported high resistance to ampicillin (94.2 %), chloramphenicol (72.8 %) and lower resistance (31.8 %) to co-trimoxazole. The NTS isolated by Ifeanyi et al. [19] were resistant to amoxicillin and cefuroxime (55.5 % each). In South-Western Nigeria, Olowe et al. [25] reported a 91.3 % resistance rate for amoxicillin and co-trimoxazole, 86.9 % for ampicillin, 82.6 % for streptomycin and 30.4 % for ciprofloxacin (Table 1). Interestingly, none of the NTS from these studies in Nigeria were resistant to ciprofloxacin, nalidixic acid or ofloxacin. Most studies in Africa have reported NTS susceptibility to fluoroquinolones [27]. Akinyemi et al. [26] isolated Salmonella serotypes that had reduced susceptibility to fluoroquinolones in Nigeria. This is a new scourge which was also reported by Boni-Cissé et al. [16] and Lunguya et al. [28] in Côte d’Ivoire and the Democratic Republic of Congo, respectively. In Côte d’Ivoire, resistance to nalidixic acid was 38 % and reduced susceptibility to ciprofloxacin was 14 %, while in the Democratic Republic of Congo, there was 15.4 % decreased susceptibility to ciprofloxacin. However, one study from northern Nigeria reported 100 % resistance of human NTS to fluoroquinolones [29]. There are some documented underlying conditions that may have contributed to such failure, which include diabetes, AIDS, chronic liver disease, aortic aneurysm surgery and spherocytosis [30]. In comparison with data from other continents of NTS serovars resistant to ciprofloxacin, Africa is second only to Asia [31]. Investigation conducted so far suggest that an alteration in gyrA is solely responsible for resistance [32]. This is attributed to a point mutation in the quinolone resistance determining region (QRDR) of the topoisomerase gene gyrA. However, in Lagos, Nigeria, it was observed that MDR NTS isolated from HIV patients was chromosomally mediated [33].
Transmission of non-typhoidal Salmonella
The transmission of NTS in Africa has not been well established. However, there has been speculation of human-to-human transmission coupled with food-borne transmission, which is said to be the predominant source of transmission [9, 34]. Kagambèga et al. [35], in their study, carried out a comparison of NTS isolated from the faeces of cattle, poultry, swine and hedgehogs to NTS isolated from humans in Burkina Faso. It was observed that S. typhimurium isolates from poultry and humans were multi-resistant to the same set of antimicrobials (ampicillin, chloramphenicol, streptomycin, sulphonamides and trimethoprim). Also, they had the same phage type DT56 and were closely related in pulsed-field gel electrophoresis (PFGE). Furthermore, S. muenster isolated from hedgehogs had similar PFGE patterns as the domestic animals, which implies that domestically produced animals and wild animals can share the same Salmonella serotypes and potentially transmit some of them to humans. A similar study in Tunisia suggested the possibility of circulating epidemic strains between contaminated animal-derived meat and humans [36]. Several risk factors have, over time, been identified as factors that can foster the transmission of NTS, which include environment (food and water, hospital-acquired infection, direct and indirect animal contact, transmission between humans) and host (age, HIV infection, malnutrition, sickle cell disease, malarial anaemia, schistosomiasis and recent antimicrobial use) [37].
Tracking the transmission of emerging multidrug-resistant Salmonella serovars
With the application of recent sequencing technologies, tracking of the emergence and transmission of MDR Salmonella serovars has been made possible. Both MDR typhoidal and non-typhoidal Salmonella serovars can be tracked using whole-genome DNA sequencing. Multilocus sequencing analysis of invasive S. typhimurium from Malawi and Kenya identified a dominant type, designated ST313, which was rarely reported outside of Africa [38]. Whole-genome DNA sequences have revealed that the worldwide S. typhi population is highly clonal and probably originated from a common ancestor that moved into the human population several thousand years ago. Phylogeographical analysis of the dominant MDR H58 clade of S. typhi revealed an intercontinental (Asia–East Africa) and intracontinental (East–South Africa) transmission [39].
Difference between typhoidal and non-typhoidal Salmonella
With over 2600 different serovars, S. enterica is greatly diverse, hence the need to know the difference between typhoidal and non-typhoidal Salmonella, as both are of the same species but result in different disease manifestation. Salmonella typhimurium and S. enteritidis have a wide host specificity compared to typhoidal strains that are human host-specific. In terms of epidemiology, NTS has a global burden in contrast to typhoidal Salmonella, which is majorly endemic in developing countries, such as South-East Asia and Africa. This could be attributed to poor sanitary environment and low standard of living. Also, there are no human vaccines available for NTS, while killed whole-cell parenteral vaccine, live-attenuated oral vaccine and Vi polysaccharide capsule-based vaccine are available [22].
Typhoid fever in Africa
Epidemiology
Enteric fever (typhoid and paratyphoid fevers) is a febrile illness caused by S. typhi and Salmonella paratyphi A, B and C [40]. Worldwide, typhoid fever is an important cause of morbidity and mortality, with an estimated 16–33 million cases and 500,000–600,000 deaths annually [41]. Salmonella typhi is endemic in developing countries such as Africa, in contrast to developed countries, where the incidence is much lower and the majority of cases occur among travellers returning from endemic areas [42]. In 2002, it was estimated that a total of 408,837 cases occurred in Africa [43]. However, the exact distribution of typhoid fever in Africa is not well documented due to the non-availability of facilities capable of performing the blood culture tests essential for the diagnosis of typhoid fever and, also, typhoid fever is commonly attributed to malaria [44, 45].
Typhoid fever is transmitted through the faecal–oral route via contaminated water and food. It is prevalent among impoverished populations [46] that are overcrowded, with poor access to sanitation and are exposed to unsafe water and food, and also affects travellers bound to endemic countries [47]. Food handlers also play a prominent role in the transmission of typhoid fever [48]. Typical symptoms of enteric fever include fever, chills, hepatosplenomegaly and abdominal pain. In addition, patients may experience nausea, rash, diarrhoea or constipation and headaches [49].
Estimates of typhoid fever disease burden in Africa
Knowledge of the burden of disease is vital in order to know the effects of the disease on human health. Hence, efforts to understand the trend of typhoid fever in Africa require the need for well-planned studies. In Egypt, a study [50] was carried out to determine the population-based incidence of typhoid fever and, of 1815 patients evaluated, cultures yielded 90 (5 %) S. typhi isolates and the estimated incidence of typhoid fever was 59/100,000 persons/year. Studies on typhoid infection from Egypt are in the medium incidence range, but a study from South Africa reported a high incidence range for the South African region [43].
In Ghana, it was reported that typhoid fever was among the leading causes of outpatient illness, accounting for 0.92 % of hospital admissions [51]. Also, in Ghana, typhoid fever incidences were calculated for the period from September 2007 to November 2008, and 2.5 % of the isolates were positive for S. typhi. The frequency of typhoid fever was low among children <2 years of age (0.7 %), while among those children between 2 to <11 years of age it was 7.0 % and among children ≥11 years of age it was 4.6 % [52]. In Kenya, a study by Breiman et al. [53] reported very high rates of bacteraemic typhoid fever in urban areas in Kenya (247/100,000 per population per year). In Nigeria, a study of a 4-year cumulative prevalence of Salmonella infection in Akwa Ibom state using hospital-based data reported a high prevalence (63.8 %) of typhoid fever in both sexes and all age groups [54]. A study from Northwest Nigeria showed S. typhi to be the most frequently encountered, in 40.7 % of cases [20]. A study in Tanzania reviewed typhoid records for 5 years, with the results indicating that there was a fluctuating trend of typhoid occurrence, and the overall typhoid fever prevalence between 2003 and 2007 indicated an increasing trend from 771 to 942 cases/100,000 persons [55]. In Cameroon, a cross-sectional study was carried out to determine the prevalence of typhoid fever in 200 patients and typhoid fever was confirmed in 2.5 % [56].
The bacterium
Salmonella typhi belongs to the family Enterobacteriaceae of the general group of enteric bacteria that includes Escherichia coli and Shigella species [57]. It is a Gram-negative bacillus which is aerobic and facultative anaerobic. Serologically, S. typhi is positive to lipopolysaccharide antigen 09, 12, protein flagellar antigen Hd and capsular polysaccharide antigen Vi [46]. Salmonella typhi is human host-restricted; thus, humans are the only known natural host of S. typhi [58] and study has shown that isolates of S. typhi are highly clonally related [59].
Genomics of S. typhi
The genome of S. typhi consists of approximately 5 million base pairs (bp) encoding about 4000 genes, of which more than 200 are pseudogenes [57, 60]. A remarkable characteristic of the S. typhi genome is the presence of the pseudogenes and more than half of which are inactivated by the introduction of a stop codon. A significant number are predicted to be involved in housekeeping functions, virulence or host interactions, and this inactivation of genes which is responsible for host interactions may explain why S. typhi is human host-restricted [46].
The complete DNA sequences of two different S. typhi isolates include the S. typhi type strain Ty2 originally isolated in the early 1900s and S. typhi CT18, an MDR strain which was isolated in 1993 from a child with typhoid fever in the Mekong Delta region of Vietnam [57–59]. Salmonella typhi Ty2 was the basis for vaccine development and was the parent of mutant strains Ty21a and CVD908, which were used in trials of live-attenuated vaccines and contains no plasmids [58]. However, S. typhi CT18 with a total size of 4,809,037 bp is an example of an MDR microorganism [59]. Salmonella typhi CT18 contains two plasmids and the larger conjugative plasmid, pHCM1, is 218 kb in length and encodes resistance to chloramphenicol, ampicillin, trimethoprim, sulphonamides and streptomycin. The smaller plasmid, pHCM2, is 106.5 kb in length and is phenotypically cryptic [46, 57].
Also, Vi polysaccharide capsule production is one of the major characteristics of most S. typhi isolates and is associated with a set of genes that are situated within a gene island of 134 kb that encodes a variety of putative virulence-associated gene clusters (including the Vi) known as SPI-7 [61].
Pathogenesis and pathology of typhoid fever
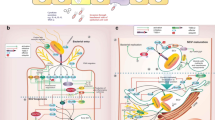
Upon the ingestion of S. typhi in contaminated food or water by the host, it passes through the stomach and invades the gut epithelium of the human host. Salmonella typhi enters the host’s system primarily through the distal ileum and is transported into the lymphatic system [62]. Attachment promoted by the bacterium may be necessary before invasion can occur and S. typhi has specialised fimbriae that adhere to the epithelium in the ileum (Peyer’s patches). Once there, they pause and continue to multiply until some critical point is attained. The critical point is most likely determined by the number of bacteria, their virulence and the host response [46]. The incubation period is usually 7 to 14 days, after which the bacteria break out into the bloodstream to invade the rest of the body. Bacteria that do not re-infect the host are naturally shed in the stool and are then ready to infect other hosts [46]. Once S. typhi invades the gut epithelium, it encounters macrophages within the gut-associated lymphoid tissue. Hence, the interaction between Salmonella and macrophages lead to an alteration in the expression of a number of host genes, such as those encoding pro-inflammatory cytokines (including IL-1, IL-6, IL-8, TNF-β, INF, GM-CSF), receptors, adhesion molecules and anti-inflammatory mediators, as well as those involved in cell death or apoptosis [62, 63].
The Vi-positive strains of S. typhi are more infectious and highly virulent compared to Vi-negative strains [46]. The initial interaction between S. typhi and the human gut is less inflammatory due to the absence of neutrophilic intestinal infiltrates in the acute phase of typhoid fever, which suggests a tendency for typhoidal Salmonella to evade aspects of the innate immune response and cause a systemic infection [64].
Pathology in the Payer’s patches include hyperplasia of lymphoid follicles, necrosis of lymphoid follicles and ulceration in the long axis of the bowel, with the possibility of perforation and haemorrhage [63].
Virulence factors of Salmonella species
Several Salmonella virulence mechanisms have been identified to describe the disease presentation, including altered flagellin gene regulation [65], altered invasion gene regulation [66], expression of the typhoid toxin [67] and expression of the virulence-associated (Vi) capsular polysaccharide [68]. Salmonella pathogenicity islands (SPIs) are responsible for the majority of the virulence factors of the bacterium [69]. The ability of Salmonella to survive and replicate within the phagosome is mediated by Salmonella pathogenicity island 2 (SPI-2), which inhibits movement of reactive oxygen intermediates and reactive nitrogen intermediates into the phagosome [70]. However, SPI-7 is the most significant pathogenicity island in S. typhi infection because it codes for the Vi antigen that is expressed on the cell surface. The Vi antigen is essential for increased virulence and severity of symptoms [71].
Most of the effector molecules associated with the pathogen’s virulence are encoded on the SPIs. The type III secretion system (T3SS) proteins encoded by two SPIs are linked with the pathogenicity at the molecular level. The T3SS structural genes positioned on SPI-1 include prgHIJK, spaMNOPQRS and invABCEFGH, as well as multiple regulatory and effector genes [69, 72]. Salmonella typhi secretes effector proteins which include, SipA, SipB, SipC, SopA, SopB, SopC, SopD, SopE, SptP, SpiC and SseF. SipA and SptP alter the actin cytoskeleton of the host cell, which is responsible for cell migration [69, 73]. Recently, Smith et al. [74] from Lagos, Nigeria reported 73 out of 76 isolates (96.1 %) to be positive for the invA, gene while 38 (50 %) possessed the sitC gene out of the 76 isolates tested. Therefore, they recommended the use of the invA gene for Salmonella detection in food samples in Nigeria. In a study by Adesiji et al. [75] on 16 NTS clinical isolates from north central Nigeria, all found to harbour the invA gene, while a study by Dione et al. [76] from Gambia tested 185 NTS for the presence of 12 virulence genes and found the genes present in at least 70 % of the isolates. Karmi [77] from Egypt reported on all NTS isolates from poultry and meat samples positive for the invA gene.
Host immune response to S. typhi
During invasive Salmonella infection, Salmonella pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) initiate the innate immune system, leading to activation and recruitment of neutrophils, dendritic cells (DCs), inflammatory monocytes and macrophages, and the production of pro-inflammatory cytokines, most notably IL-1, IL-2, IL-6 and IL-8, tumour necrosis factor (TNF)-a, and interferon (IFN)-γ [78]. Also, S. typhi-infected macrophages can be stimulated by producing lysosomal enzymes, reactive nitrogen intermediates, reactive oxygen intermediates and other antimicrobial peptides in order to destroy or limit the replication of Salmonella [79].
Clearance of S. typhi from tissues requires the CD28-dependent activation of CD4+, T cell receptor (TCR)-α β T cells and is controlled by major histocompatibility complex (MHC) class II genes. DCs antigen-presenting cells process the antigen and present it on MHC class II molecules to naive T cells [72].
Diagnosis of S. typhi
Salmonella typhi or S. paratyphi can be detected either by culture of the organism or by the serological methods using serum or urine samples. The organism may be cultured from blood, bone marrow, stool or urine [63]. The gold standard method for confirming typhoid fever is an isolation of S. typhi from bone marrow and this requires equipment and trained laboratory personnel. However, they are not available in primary healthcare facilities and hospitals in the developing world [80]. In 2011, Keddy et al. [81] evaluated three diagnostic methods for detecting antibodies to S. typhi and used blood culture as the standard for comparison. Patients were recruited for the study from two sub-Saharan African sites [81]. It was reported that all typhoid rapid antibody tests performed poorly compared with blood culture and it was concluded that such tests may be useful only during emergencies such as outbreaks and was not recommended for routine care settings in sub-Saharan Africa. However, Smith et al. [82] recommended the use of both S. typhi culture from faecal samples and the Widal test for diagnosis. In Africa, the most common diagnosis of S. typhi is serological Widal’s test [83] and typhoid fever in most developing countries is a disease of over- and under-diagnosis [84]. Rapid tests used in the diagnosis of typhoid fever include Typhidot, TUBEX and Multi-Test Dip-S-Ticks to detect immunoglobulin G (IgG) and IgM, (IgM) and IgG, respectively. Olsen et al. [85] reported the sensitivity and specificity of these kits to be 89 and 53 % for Multi-Test Dip-S-Ticks, 79 and 89 % for Typhidot, 78 and 89 % for TUBEX, and 64 and 76 % for Widal testing in hospital findings. Also, Ismail [86] reported the evaluation of Typhidot specificity and sensitivity to be 75 and 95 %, respectively, in clinical settings. In addition, Krishna et al. [87] reported in a previous study that the Typhidot (IgM) test is a reliable and rapid test for the diagnosis of typhoid fever, with a sensitivity of 100 % and a specificity of 95.5 %. This is in consonance with the report of Smith et al. [88], which revealed 97.8 % specificity. Recently, a systematic review on the diagnosis of typhoid fever was performed by Storey et al. [89] and it was reported that blood culture (70 %) was the most frequently used test, while the Widal test accounted for only 7 %. This implies that the Widal test is not a method of assay in other clans except Africa. However, they recommended the approval of a composite reference standard to detect typhoid fever which can enhance the estimation of diagnostic validity.
Culture
The liquid and solid media that are suitable for the isolation of S. typhi and salmonellosis include strontium selenite broth, which is better than selenite F broth for the isolation of S. typhi; Salmonella–Shigella agar has been found to work better than xylose–lysine deoxycholate agar and modified bismuth sulphate agar, which is better than deoxycholate agar for the growth of Salmonella spp. The organism is less frequently isolated from urine, and culture of bone marrow may yield more isolates of the organism even when it cannot be obtained from blood, stool or urine [63].
Serological tests
The Widal test, which measures the antibody titres to the O (somatic) and H (flagella) antigens, is relied upon widely in Africa, although serological tests are of little value because they are neither sensitive nor specific and are not confirmed suitably for widespread adoption. An enzyme immunoassay for the rapid detection of S. typhi-specific IgM and IgG antibodies has been reported to be sensitive but has not been confirmed for widespread implementation. Smith et al. [88] employed the classical Widal test and rapid latex agglutination assay for typhoid fever diagnosis. In comparison, the simple and rapid test was more sensitive to the Widal test. The classification for the genus Salmonella by Kauffman was recognised on the basis of the serologic identification of O (somatic), which forms the immunodominant part of LPS and H (flagellar) antigens, the filamentous portion of the bacterial flagellum. Each serotype was considered a separate species based on the Kauffman–White scheme [90].
Antimicrobial resistance of S. typhi
Antimicrobial resistance in developing countries is a major public health problem and the emergence of S. typhi MDR strains has been the main challenge to healthcare systems and is, therefore, of major concern [42], whereby the effective treatment for the disease is reduced, leading to complications and death. Multidrug resistance to the traditional first-line antimicrobial agents ampicillin, chloramphenicol and trimethoprim–sulphamethoxazole is common among S. typhi [91, 92] and MDR strains resistance to ampicillin, chloramphenicol and trimethoprim has been encoded by plasmids belonging to the H1 incompatibility group (incH1) [42]. In Africa, antimicrobial susceptibility data are insufficient and this issue is challenging and of great concern, since treatment with proper antimicrobial drugs is essential for the effective treatment of S. typhi. In Nigeria, it was reported in a study that S. typhi strains were resistant to at least three antibiotics, giving a prevalence of 80.7 % [93, 94], i.e. a high prevalence of MDR S. typhi. Also, Akinyemi et al. [95] confirmed the circulation of multidrug resistance in S. typhi isolated from patients with typhoid fever complications in Lagos, Nigeria. In Ghana, Mills-Robertson et al. [96] reported multidrug resistance in 30 of 58 isolates based on their resistance to three out of the five antibiotics tested. MDR S. typhi was also reported in a study by Lunguya et al. [28] in the Democratic Republic of Congo, in Togo [97] and on the Malawi–Mozambique border [98].
The emergence of MDR strains that were resistant to the first-line antimicrobial drugs was made known in the 1980s, after which stains resistant to fluoroquinolones emerged in the 1990s [99]. Resistance to nalidixic acid (minimum inhibitory concentration > 256 mg/l) serves as a marker for the detection of reduced susceptibility of Salmonella spp. to fluoroquinolones. Resistance to fluoroquinolones in S. typhi has been linked to be mediated by chromosomal mutations, in the target proteins, DNA gyrase, encoded by gyrA and gyrB or by plasmid-mediated resistance [100] and antimicrobial resistance, notably to fluoroquinolones, is on the increase [101]. Fluoroquinolones act by inhibiting the topoisomerase enzymes, DNA gyrase and topoisomerase IV, and a number of resistance mechanisms have been documented, such as point mutations that result in amino acid substitutions in the topoisomerases, reduced outer membrane permeability, increased efflux of antibiotics and the plasmid-encoded Qnr genes [101]. In Africa, Keddy et al. [102] reported fluoroquinolone-resistant typhoid fever in South Africa. Also, Kariuki et al. [44] reported the trends in resistance to quinolones and the distribution of drug resistance phenotypes among S. typhi isolates from three surveillance periods.
Multidrug-resistant S. typhi H58 clone
The MDR S. typhi H58 clone has become prevalent in the Indian subcontinent, South-East Asia and has also spread to Eastern Africa. This clone acquired a large conjugative incH1 pST6 plasmid encoding resistance to co-trimoxazole, ampicillin and chloramphenicol. MDR S. typhi showed reduced susceptibility to ciprofloxacin and also turned out to be resistant to quinolones as far back as the 1990s [103]. In Africa, Feasey et al. [104] and Kariuki et al. [44] reported an increase in the incidence of the MDR H58 lineage of S. typhi in Blantyre, Malawi, and H58 multidrug resistance in Kenya, respectively.
Vaccination
Currently, typhoid fever can be treated with antibiotic drugs, but the alarming rates of antibiotic resistance calls for vaccination in high-risk populations with typhoid fever [105]. Hence, the World Health Organization (WHO) recommends the use of two licensed vaccines, Ty21a and Vi [105]. Ty21a is an oral live-attenuated vaccine and Vi is an injectable capsular polysaccharide vaccine. Both vaccines have been proven to be safe and effective in several clinical trials and field settings [106].
Treatment
Treatment with fluoroquinolones, azithromycin and third-generation cephalosporin drugs is the main treatment of choice [46], with chloramphenicol used in regions where there are susceptible strains. In 2003, the WHO recommended the treatment of uncomplicated typhoid fever to include ciprofloxacin, chloramphenicol, amoxicillin and co-trimoxazole [105].
Conclusion
The current status of Salmonella infections in Africa calls for new strategies and resources in order to curb infections. The introduction of simple, more specific methods of diagnosis at an affordable cost will be of immense benefit to healthcare givers in tackling Salmonella infections in Africa. The prevention control strategy should include health education, sanitation improvements and good water quality. In the area of increasing antibiotic resistance to Salmonella typhi, vaccination should be considered for both vulnerable children and adults in order to prevent the disease, since the disease burden is high in Africa.
References
(2006) Salmonella. In: Greenwood D, Slack RCB, Peutherer JF (eds) Medical microbiology. A guide to microbial infections, pathogenesis, immunity, laboratory diagnosis and control, 16th edn. Elsevier Science Limited, Churchill Livingstone, pp 250–259
Grimont PAD, Weill FX (2007) Antigenic formulae of the Salmonella serovars, 9th edn. World Health Organization Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur, Paris, France
Rotger R, Casadesús J (1999) The virulence plasmids of Salmonella. Int Microbiol 2:177–184
Su LH, Chiu CH (2007) Salmonella: clinical importance and evolution of nomenclature. Chang Gung Med J 30(3):210–219
Pui CF, Wong WC, Chai LC, Robin T, Ponniah J, Sahroni NHM et al (2011) Salmonella: a foodborne pathogen. Int Food Res J 18:465–473
Haeusler GM, Curtis N (2013) Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol 764:13–26
Bisi-Johnson MA, Obi CL (2012) Escherichia coli and Salmonella species: molecular landscape and therapeutic considerations: a review. Adv Med Sci 1(1):1–16
Akyala AI, Alsam S (2015) Extended spectrum beta lactamase producing strains of salmonella species—a systematic review. J Microbiol Res 5(2):57–70
Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J et al (2006) Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol 55:585–591
Majowicz SE, Musto J, Scallan E (2010) International collaboration on enteric disease “Burden of Illness” studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50(6):882–889
Mandomando I, Macete E, Sigaúque B, Morais L, Quintó L, Sacarlal J et al (2009) Invasive non-typhoidal Salmonella in Mozambican children. Trop Med Int Health 14(12):1467–1474
Kariuki S, Okoro C, Kiiru J, Njoroge S, Omuse G, Langridge G et al (2015) Ceftriaxone-resistant Salmonella enterica serotype typhimurium sequence type 313 from Kenyan patients is associated with the blaCTX-M-15 gene on a novel IncHI2 plasmid. Antimicrob Agents Chemother 59:3133–3139
Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA et al (2010) Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis 16(9):1448–1451
Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, Dramaix-Wilmet M et al (2001) Community-acquired bacteremia among hospitalized children in rural central Africa. Int J Infect Dis 5:180–188
Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M et al (2010) Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis 4(3):e621
Boni-Cissé C, Meité S, Faye-Ketté H, Houedanou C, Timité-Konan M, Kalpi C et al (2012) Serotypes and antibiotypes of Salmonella isolated at the University Teaching Hospital of Yopougon, Abidjan, Côte d’Ivoire from 2005 to 2009. J Microbiol Antimicrob 4(2):40–44
Mohamed MEM, Suelam IIA (2010) Isolation of non-typhoid Salmonella from humans and camels with reference to its survival in abattoir effluents. Glob Vet 5(6):356–361
Labi AK, Obeng-Nkrumah N, Addison NO, Donkor ES (2014) Salmonella blood stream infections in a tertiary care setting in Ghana. BMC Infect Dis 14:3857
Ifeanyi CI, Bassey BE, Ikeneche NF, Al-Gallas N (2014) Molecular characterization and antibiotic resistance of Salmonella in children with acute gastroenteritis in Abuja, Nigeria. J Infect Dev Ctries 8(6):712–719
Abdullahi B, Abdulfatai K, Wartu JR, Mzungu I, Muhammad HID, Abdulsalam AO (2014) Antibiotics susceptibility patterns and characterization of clinical Salmonella serotypes in Katsina State, Nigeria. Afr J Microbiol Res 8:915–921
Glynn JR, Palmer SR (1992) Incubation period, severity of disease, and infecting dose: evidence from a Salmonella outbreak. Am J Epidemiol 136(11):1369–1377
Gal-Mor O, Boyle EC, Grassl GA (2014) Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol 5:391
Oundo JO, Muli F, Kariuki S, Waiyaki PG, Iijima Y, Berkley J et al (2002) Non-typhi Salmonella in children with severe malaria. East Afr Med J 79(12):633–639
Mengistu G, Mulugeta G, Lema T, Aseffa A (2014) Prevalence and antimicrobial susceptibility patterns of Salmonella serovars and Shigella species. J Microbial Biochem Technol S2:006
Olowe OA, Okanlawon BM, Olowe RA, Adedosu OT, Olayemi AB (2007) Multiple drug resistant pattern of Salmonella typhimurium infections in Osogbo, South Western Nigeria. J Am Sci 3(4):40–44
Akinyemi KO, Bamiro BS, Coker AO (2007) Salmonellosis in Lagos, Nigeria: incidence of Plasmodium falciparum-associated co-infection, patterns of antimicrobial resistance, and emergence of reduced susceptibility to fluoroquinolones. J Health Popul Nutr 25(3):351–358
Reda AA, Seyoum B, Yimam J, Andualem G, Fiseha S, Vandeweerd JM (2011) Antibiotic susceptibility patterns of Salmonella and Shigella isolates in Harar, Eastern Ethiopia. J Infect Dis Immun 3:134–139
Lunguya O, Lejon V, Phoba MF, Bertrand S, Vanhoof R, Verhaegen J et al (2012) Salmonella typhi in the Democratic Republic of the Congo: fluoroquinolone decreased susceptibility on the rise. PLoS Negl Trop Dis 6(11):e1921
Ishaku AA, Katsa M, Yakubu H, Habibu T, Daniel A, Solomon A (2013) Incidence and antimicrobial resistance pattern of some clinical isolate of Salmonella Typhi in Akwanga, Nasarawa State, Nigeria. J Med Biomed Sci 4:1–5
Humphries RM, Fang FC, Aarestrup FM, Hindler JA (2012) In vitro susceptibility testing of fluoroquinolone activity against Salmonella: recent changes to CLSI standards. Clin Infect Dis 55(8):1107–1113
Marshall B, Kadangs C (2014) Public health threat agent: Salmonella. APUA Newsl 32(3):1–21
Piddock LJV (2002) Fluoroquinolone resistance in Salmonella serovars isolated from humans and food animals. FEMS Microbiol Rev 26:3–16
Adeleye A, Smith S, Akanmu S, Bamiro S, Sobande O, Igbinosum E et al (2008) Chromosomally mediated antibiotic resistance in non-typhoidal Salmonellae isolated from HIV patients in Lagos. West Indian Med J 57(5):519–520
Pegues DA, Miller SI (2010) Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R (eds) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 7th edn. Elsevier, Philadelphia, pp 2887–2903
Kagambèga A, Lienemann T, Aulu L, Traoré AS, Barro N, Siitonen A et al (2013) Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonella isolates. BMC Microbol 13:253
Abbassi-Ghozzi I, Hammami S, Aissa RB, Martinez-Urtaza J, Boudabous A, Gtari M (2012) Pulsed-field gel electrophoresis, plasmid profile and antimicrobial resistance pattern of Salmonella typhimurium isolated from human and retail meats. Afr J Microbiol Res 6(22):4680–4686
Morpeth SC, Ramadhani HO, Crump JA (2009) Invasive non-typhi Salmonella disease in Africa. Clin Infect Dis 49:606–611
Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA et al (2009) Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19(12):2279–2287
Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA et al (2015) Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47:632–639
Crump JA, Mintz ED (2010) Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50(2):241–246
DeRoeck D, Jodar L, Clemens J (2007) Putting typhoid vaccination on the global health agenda. N Engl J Med 357:1069–1071
Threlfall EJ (2002) Antimicrobial drug resistance in Salmonella: problems and perspectives in food- and water-borne infections. FEMS Microbiol Rev 26:141–148
Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82:346–353
Kariuki S, Revathi G, Kiiru J, Mengo DM, Mwituria J, Muyodi J et al (2010) Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J Clin Microbiol 48(6):2171–2176
Reddy EA, Shaw AV, Crump JA (2010) Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10:417–432
Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ (2002) Typhoid fever. N Engl J Med 347(22):1770–1782
Whitaker JA, Franco-Paredes C, del Rio C, Edupuganti S (2009) Rethinking typhoid fever vaccines: implications for travelers and people living in highly endemic areas. J Travel Med 16:46–52
Smith SI, Agomo CO, Bamidele M, Opere BO, Aboaba OO (2010) Survey of food handlers in Bukas (a type of local restaurant) in Lagos, Nigeria about typhoid fever. Health 2(08):951–956
Buckle GC, Walker CL, Black RE (2012) Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2(1):010401
Srikantiah P, Girgis FY, Luby SP, Jennings G, Wasfy MO, Crump JA et al (2006) Population-based surveillance of typhoid fever in Egypt. Am J Trop Med Hyg 74(1):114–119
Sory E (2009) The health sector in Ghana. Facts and figures. Ghana Health Service, Accra, Ghana, p 31
Marks F, Adu-Sarkodie Y, Hünger F, Sarpong N, Ekuban S, Agyekum A et al (2010) Typhoid fever among children, Ghana. Emerg Infect Dis 16(11):1796–1797
Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB et al (2012) Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 7(1):e29119
Uttah EC, Osim SE, Etta H, Ogban E, Okon NEE (2013) Four-year longitudinal assessment of the prevalence of typhoid fever among those attending the General Hospital Etinan, Nigeria. Int J Sci Res Pub 3(7):150–153
Malisa A, Nyaki H (2010) Prevalence and constraints of typhoid fever and its control in an endemic area of Singida region in Tanzania: lessons for effective control of the disease. J Public Health Epidemiol 2(5):93–99
Nsutebu EF, Martins P, Adiogo D (2003) Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Trop Med Int Health 8(6):575–578
Baker S, Dougan G (2007) The genome of Salmonella enterica serovar Typhi. Clin Infect Dis 45:S29–S33
Deng W, Liou SR, Plunkett G 3rd, Mayhew GF, Rose DJ, Burland V et al (2003) Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J Bacteriol 185(7):2330–2337
Parkhill J, Dougan G, James KD, Thomson NR, Pickard D, Wain J et al (2001) Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852
McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A et al (2004) Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet 36(12):1268–1274
Pickard D, Wain J, Baker S, Line A, Chohan S, Fookes M et al (2003) Composition, acquisition, and distribution of the Vi exopolysaccharide-encoding Salmonella enterica pathogenicity island SPI-7. J Bacteriol 185:5055–5065
House D, Bishop A, Parry C, Dougan G, Wain J (2001) Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis 14:573–578
Singh S (2001) Symposium: typhoid fever. Pathogenesis and laboratory diagnosis. J Indian Acad Clin Med 2(1):17–20
Raffatellu M, Wilson RP, Winter SE, Bäumler AJ (2008) Clinical pathogenesis of typhoid fever. J Infect Dev Ctries 2(4):260–266
Winter SE, Raffatellu M, Wilson RP, Rüssmann H, Bäumler AJ (2008) The Salmonella enterica serotype typhi regulator TviA reduces interleukin-8 production in intestinal epithelial cells by repressing flagellin secretion. Cell Microbiol 10:247–261
Winter SE, Winter MG, Thiennimitr P, Gerriets VA, Nuccio SP, Rüssmann H et al (2009) The TviA auxiliary protein renders the Salmonella enterica serotype Typhi RcsB regulon responsive to changes in osmolarity. Mol Microbiol 74:175–193
Song J, Gao X, Galán JE (2013) Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature 499:350–354
Wilson RP, Raffatellu M, Chessa D, Winter SE, Tükel C, Bäumler AJ (2008) The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol 10:876–890
Ruby T, McLaughlin L, Gopinath S, Monack D (2012) Salmonella’s long-term relationship with its host. FEMS Microbiol Rev 36:600–615
Chakravortty D, Hansen-Wester I, Hensel M (2002) Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med 195:1155–1166
Tran QT, Gomez G, Khare S, Lawhon SD, Raffatellu M, Bäumler AJ et al (2010) The Salmonella enterica serotype Typhi Vi capsular antigen is expressed after the bacterium enters the ileal mucosa. Infect Immun 78:527–535
Kaur J, Jain SK (2012) Role of antigens and virulence factors of Salmonella enterica serovar Typhi in its pathogenesis. Microbiol Res 167(4):199–210
McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V (2009) Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol 12:117–124
Smith SI, Fowora MA, Atiba A, Anejo-Okopi J, Fingesi T, Adamu ME et al (2015) Molecular detection of some virulence genes in Salmonella spp isolated from food samples in Lagos, Nigeria. Anim Vet Sci 3(1):22–27
Adesiji YO, Deekshit VK, Karunasagar I (2014) Antimicrobial-resistant genes associated with Salmonella spp. isolated from human, poultry, and seafood sources. Food Sci Nutr 2(4):436–442
Dione MM, Ikumapayi U, Saha D, Mohammed NI, Adegbola RA, Geerts S et al (2011) Antimicrobial resistance and virulence genes of non-typhoidal Salmonella isolates in the Gambia and Senegal. J Infect Dev Ctries 5:765–775
Karmi M (2013) Detection of virulence gene (invA) in Salmonella isolated from meat and poultry products. Int J Genet 3:07–12
Kupz A, Guarda G, Gebhardt T, Sander LE, Short KR, Diavatopoulos DA et al (2012) NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8(+) T cells. Nat Immun 13:162–169
Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC (2000) Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192:227–236
Wain J, Hosoglu S (2008) The laboratory diagnosis of enteric fever. J Infect Dev Ctries 2:421–425
Keddy KH, Sooka A, Letsoalo ME, Hoyland G, Chaignat CL, Morrissey AB et al (2011) Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ 89:640–647. doi:10.2471/BLT.11.087627
Smith SI, Odunukwe NN, Niemogha MT, Ahmed AO, Efienemokwu CA, Otuonye MN et al (2004) Diagnostic methods for typhoid fever in Nigeria. Br J Biomed Sci 61(4):179–181
Mweu E, English M (2008) Typhoid fever in children in Africa. Trop Med Int Health 13(4):532–540
Otegbayo JA (2005) Typhoid fever: the challenges of medical management. Ann Ibadan Postgrad Stud 3(1):52–54
Olsen SJ, Pruckler J, Bibb W, Nguyen TM, Tran MT, Nguyen TM et al (2004) Evaluation of rapid diagnostic tests for typhoid fever. J Clin Microbiol 42(5):1885–1889
Ismail A (2000) New advances in the diagnosis of typhoid and detection of typhoid carriers. Malays J Med Sci 7(2):3–8
Krishna S, Desai S, Anjana VK, Paranthaaman RG (2011) Typhidot (IgM) as a reliable and rapid diagnostic test for typhoid fever. Am Trop Med Public health 4:42–44
Smith SI, Bamidele M, Fowora M, Goodluck HT, Omonigbehin EA, Akinsinde KA et al (2011) Application of a point-of-care test for the serodiagnosis of typhoid fever in Nigeria and the need for improved diagnostics. J Infect Dev Ctries 5(7):520–526
Storey HL, Huang Y, Crudder C, Golden A, de los Santos T, Hawkins K (2015) A meta-analysis of typhoid diagnostic accuracy studies: a recommendation to adopt a standardized composite reference. PLoS One 10(11), e0142364
Brenner FW, Villar RG, Angulo FJ, Tauxe R, Swaminathan B (2000) Salmonella nomenclature. J Clin Microbiol 38(7):2465–2467
Ugboko H, De N (2014) Mechanisms of antibiotic resistance in Salmonella typhi. Int J Curr Microbiol App Sci 3(12):461–476
Crump JA, Mintz ED (2010) Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50(2):241–246
Akinyemi KO, Coker AO (2007) Trends of antibiotic resistance in Salmonella enterica serovar Typhi isolated from hospitalized patients from 1997 to 2004 in Lagos, Nigeria. Indian J Med Microbiol 25:436–437
Makanjuola BO, Bakare RA, Fayemiwo SA (2012) Quinolone and multidrug resistant Salmonella typhi in Ibadan, Nigeria. Int J Trop Med 7(3):103–107
Akinyemi KO, Smith SI, Oyefolu AO, Coker AO (2005) Multidrug resistance in Salmonella enterica serovar Typhi isolated from patients with typhoid fever complications in Lagos, Nigeria. Public Health 119(4):321–327
Mills-Robertson F, Addy ME, Mensah P, Crupper SS (2002) Molecular characterization of antibiotic resistance in clinical Salmonella typhi isolated in Ghana. FEMS Microbiol Lett 215:249–253
Dagnra AY, Akolly K, Gbadoe A, Aho K, David M (2007) Emergence of multidrug resistant Salmonella strains in Lome (Togo). Med Mal Infect 37:266–269
Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS et al (2012) Multidrug-resistant typhoid fever with neurologic findings on the Malawi–Mozambique border. Clin Infect Dis 54(8):1100–1106
Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL (2015) Typhoid fever. Lancet 385:1136–1145
Slayton RB, Date KA, Mintz ED (2013) Vaccination for typhoid fever in sub-Saharan Africa. Hum Vaccin Immunother 9(4):903–906
Turner AK, Nair S, Wain J (2006) The acquisition of full fluoroquinolone resistance in Salmonella Typhi by accumulation of point mutations in the topoisomerase targets. J Antimicrob Chemother 58(4):733–740
Keddy KH, Smith AM, Sooka A, Ismail H, Oliver S (2010) Fluoroquinolone-resistant typhoid, South Africa. Emerg Infect Dis 16(5):879–880
Baltazar M, Ngandjio A, Holt KE, Lepillet E, Pardos de la Gandara M, Collard JM et al (2015) Multidrug-resistant Salmonella enterica serotype Typhi, Gulf of Guinea Region, Africa. Emerg Infect Dis 21(4):655–659
Feasey NA, Gaskell K, Wong V, Msefula C, Selemani G, Kumwenda S et al (2015) Rapid emergence of multidrug resistant, H58-lineage Salmonella typhi in Blantyre, Malawi. PLoS Negl Trop Dis 9(4):e0003748
World Health Organization (WHO) (2003) The diagnosis, treatment and prevention of typhoid fever. WHO, Geneva
Verma R, Bairwa M, Chawla S, Prinja S, Rajput M (2011) New generation typhoid vaccines: an effective preventive strategy to control typhoid fever in developing countries. Hum Vaccin 7:883–885
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby declare that there is no conflict of interest.
Ethical approval
This article does not contain any study that required ethical approval.
Rights and permissions
About this article
Cite this article
Smith, S.I., Seriki, A. & Ajayi, A. Typhoidal and non-typhoidal Salmonella infections in Africa. Eur J Clin Microbiol Infect Dis 35, 1913–1922 (2016). https://doi.org/10.1007/s10096-016-2760-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2760-3