Abstract
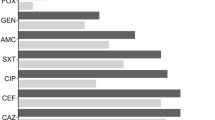
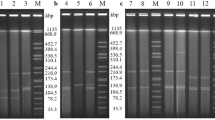
Plasmid-mediated AmpC (pAmpC) and ESBL co-production was detected in Escherichia coli a major etiologic agent of urinary tract infection. Isolates resistant to cefoxitin by CLSI methodology were tested for pAmpC beta-lactamase using phenylboronic acid and ESBLs by combined disk diffusion method. pAmpC/ESBL genes were characterized by PCR and sequencing. Transconjugation experiments were done to study the transfer of pAmpC and ESBL production from clinical isolates as donor to E. coli J53 AziR as recipient. Incompatibility groups of transmissible plasmids were classified by PCR-based replicon typing (PBRT). Among 148 urine culture positive isolates, E. coli was reported in 39.86 % (59/148), with 93.22 % (55/59) of cefoxitin resistance. pAmpC production was detected in 25, with varied distribution of blaCMY-2 and blaDHA-1type genes alone (n = 13 and 7 respectively) or in combination (n = 5). ESBL co-production was observed in 88 % (22/25) of pAmpC producing isolates with predominance of blaTEM (n = 20). Twenty-three transconjugants showed transmission of pAmpC-and ESBL-resistant genes with co-carriage of blaCMY-2 and blaTEM (n = 15) in plasmids of IncF type (n = 9) being predominant, followed by IncI1 (n = 4) and IncH1 (n = 2) in combination. All clinical isolates were clonally diverse. Resistance against different beta-lactams in uropathogenic E. coli has been an emerging concern in resource- poor countries such as India. Knowledge on the occurrence of AmpC beta-lactamases and ESBL amongst this pathogen and its transmission dynamics may aid in hospital infection control.

Similar content being viewed by others
References
Akram M, Shahid M, Khan AU (2007) Etiology and antibiotic resistance pattern of community-acquired urinary tract infection in JNMC Hospital Aligarh India. Ann Clin Microbiol Antimicrob 6:4–10
Nicolle LE (2007) Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin N Am 35(1):1–12
Gales AC, Jones RN, Gordon KA et al (2000) Activity and spectrum of 22 antimicrobial agents tested against urinary tract pathogens in hospitalised patients in Latin America: report from the second year of the SENTRY Antimicrobial Surveillance Program (1998). J Antimicrob Chemother 45:295–303
Gupta K, Sahm DF, Mayfield D et al (2001) Antimicrobial resistance among uropathogens that cause community-acquired urinary tract infections in women: a nationwide analysis. Clin Infect Dis 33:89–94
Pérez-Pérez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40(6):2153–2162
Mammeri H, Nazic H, Naas T et al (2004) AmpC β-Lactamase in an Escherichia coli Clinical isolate confers resistance to expanded-spectrum cephalosporins. Antimicrob Agents Chemother 48(10):4050–4053
Taneja N, Rao P, Arora J et al (2008) Occurrence of ESBL & Amp-C β-lactamases and susceptibility to newer antimicrobial agents in complicated UTI. Indian J Med Res 127:85–88
Manoharan A, Sugumar M, Kumar A et al (2012) Phenotypic and molecular characterization of AmpC βlactamases among Escherichia coli Klebsiella spp & Enterobacter spp from five Indian medical centers. Indian J Med Res 135:359–364
Mohamudha PR, Harish BN, Parija SC (2012) Molecular description of plasmid-mediated AmpC β-lactamases among nosocomial isolates of Escherichia coli & Klebsiella pneumoniae from six different hospitals in India. Indian J Med Res 135:114–119
Chakraborty A, Adhikari P, Shenoy S et al (2014) Characterization of Plasmid mediated AmpC producing Escherechia coli clinical isolates from tertiary care hospital in South India. Indian J Pathol Microbiol 57(2):255–258
Haenni M, Châtre P, Madec JY (2014) Emergence of Escherichiacoli producing extended-spectrum AmpCβ-lactamases(ESAC)in animals. Frontiers Microbiol 5(53):1–7
Thomson KS (2001) Controversies about extended-spectrum and AmpC β-lactamases. Emerg Infect Dis 7:333–336
Pfeifer Y, Cullik A, Witte W (2010) Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol 300:371–379
Swaminathan B, Barrett T, Hunter SB et al (2001) The molecular subtyping network for foodborne disease surveillance in the United States. Emerg Infect Dis 7:382–389
Hanson ND (2003) AmpC β-lactamases: what do we need to know for the future? J Antimicrob Chemother 52:2–4
Papanicolaou GA, Medeiros AA, Jacoby GA (1990) Novel plasmid mediated β-lactamases(MIR-1) conferring resistance to oxyimino and α methoxy β-lactams in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother 34:2200–2209
Black JA, Moland ES, Thomson KS (2005) AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β –lactamases. J Clin Microbiol 43:3110–3113
Myer K (2001) Methods in biochemical identification of bacteria. Myer’s and Koshi’s manual of diagnostic procedures in medical microbiology and immunology/serology, 2nd ed. Department of Clinical Microbiology, Christian Medical College, Vellore, pp 195–202
CLSI; Clinical and Laboratory Standards Institute (2011) Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne PA
Coudron PE (2005) Inhibitor-based methods for detection of plasmid-mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli and Proteus mirabilis. J Clin Microbiol 43:4163–4167
Kado CI, Liu ST (1981) Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol 145(3):1365–1373
MendoncaN LD, Castro AP et al (2006) ctxm-15, oxa-30 and tem-1 producing Escherechia coli in two Portugese regions. J Antimicrob Chemother 57:1014–1016
Carattoli A, Bertini A, Villa L et al (2005) Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228
Sasirekha B, Shivakumar S (2012) Occurrence of plasmid-mediated AmpC b-lactamases among Escherichia coli and Klebsiella pneumoniae clinical isolates in a tertiary care hospital in Bangalore. Indian J Microbiol 52(2):174–179
Dhanashree B, Mallya SP (2012) Molecular typing of enteropathogenic Escherichia coli from diarrheagenic stool samples. J Clin Diagn Res 6(3):400–404
Farshad S, Emamghorashi F (2009) The prevalence of virulence genes of E. coli strains isolated from children with urinary tract infection. Saudi J Kidney Dis Transpl 20:613–617
Kaluzna M, Ferrante P, Sobiczewski P, Scortichini M (2010) Characterization and genetic diversity of pseudomonas syringae from stone fruits and hazelnut using repetitive-pcr and mlst. J Plant Pathol 92:781–787
Jacoby GA (2009) AmpC betalactamases. Clin Microbiol Rev 22(1):161–182
Singhal S, Mathur T, Khan S et al (2005) Evaluation of methods for AmpC β-lactamase in Gram-negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol 23:120–124
Gupta V (2007) An update on newer betalactamases. Indian J Med Res 126(5):417–427
Ratna AK, Menon I, Kapur I et al (2003) Occurrence and detection of AmpC β-lactamases at a referral hospital in Karnataka. Indian J Med Res 118:29–32
Hemalatha PM, Sekar U et al (2007) Detection of AmpC β-lactamases production in Escherichia coli and Klebsiella by an inhibitor based method. Indian J Med Res 126:220–223
Arora S, Bal M (2005) AmpC b-lactamase producing bacterial isolates from Kolkata hospital. Indian J Med Res 122:224–233
Mata C, Miró E, Alvarado A et al (2012) Plasmid typing and genetic context of AmpC βlactamases in Enterobacteriaceae lacking inducible chromosomal AmpC genes: findings from a Spanish hospital 1999–2007. J Antimicrob Chemother 67:115–122
Alonso N, Miro E, Pascual V et al (2016) Molecular characterisation of acquired and overproduced chromosomal blaAmpC in Escherichia coli clinical isolates. Int J Antimicrob Agents 47:62–68
Ahmed SF, Ali MMM, Mohamed ZK et al (2014) Fecal carriage of extended-spectrum β-lactamases and AmpC-producing Escherichia coli in a Libyan community. Ann Clin Microbiol Antimicrob 13:22
Sallem RB, Slama KB, Rojo-Bezares B et al (2014) IncI1 Plasmids carrying blaCTX-M-1 or blaCMY-2 genes in Escherichia coli from healthy humans and animals in Tunisia. Microb Drug Resist. doi:10.1089/mdr.2013.0224
Galán-Sánchez F, Aznar-Marín P, Marín-Casanova P et al (2014) Diversity of bla genes and low incidence of CTX-M in plasmid-mediated AmpC-producing Escherichia coli clinical isolates. APMIS 122(9):796–799
Bajaj P, Singh NS, Kanaujia PK et al (2015) Distribution and molecular characterization of genes encoding CTX-M and AmpC β-lactamases in Escherichia coli isolated from an Indian urban aquatic environment. Sci Total Environ 505:350–356
Ibrahim DR, Dodd CE, Stekel DJ et al (2016) Multidrug resistant, extended spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from a dairy farm. FEMS Microbiol Ecol 92 10.1093/femsec/fiw01
Acknowledgment
The authors would like to thank Professor Nandita Basu, Director, School of Tropical Medicine, Kolkata, West Bengal, India and Professor Bibhuti Saha, Head, Department of Tropical Medicine, School of Tropical Medicine, Kolkata, West Bengal, India for their kind support to successfully carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There was no extramural funding support for this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the institutional ethical committee.
Informed consent
Informed consent was obtained from all individual participants included in the study
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Ghosh, B., Mukherjee, M. Emergence of co-production of plasmid-mediated AmpC beta-lactamase and ESBL in cefoxitin-resistant uropathogenic Escherichia coli . Eur J Clin Microbiol Infect Dis 35, 1449–1454 (2016). https://doi.org/10.1007/s10096-016-2683-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2683-z