Abstract
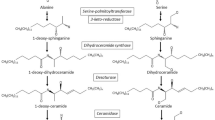
Hepatic steatosis affects disease progression in patients with chronic hepatitis C virus (HCV) infection. We investigated the plasma sphingolipid profile in patients with chronic hepatitis C (CHC) and whether there was an association between HCV-related steatosis and plasma sphingolipids. We used high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) to analyze plasma sphingolipids in 120 interferon-naïve, non-diabetic, and non-obese CHC patients. Hepatic steatosis was defined as ≥5 % hepatocytes with fat based on histopathological analysis. Blood biochemical indicators and HCV load and genotype were also determined. Thirty-six (30.0 %) of 120 patients presented with hepatic steatosis Grades 1–3. Forty-four plasma sphingolipids were detected. Plasma sphingomyelin (SM) (d18:1/22:0) and ceramide (Cer) (d18:1/24:0)-1-P correlated with steatosis grade (r = 0.22, p = 0.015; r = −0.23, p = 0.012, respectively). SM (d18:1/22:0) [odds ratio (OR) = 1.12] and Cer (d18:1/24:0)-1-P (OR = 0.88) were independent factors for the presence of hepatic steatosis in CHC patients. The area under the curve (AUC) of SM (d18:1/22:0) and Cer (d18:1/24:0)-1-P was 0.637 and 0.638, respectively, to identify the presence of steatosis. Further analysis for genotype 2 CHC showed that only SM (d18:1/22:0) was independently linked to steatosis (OR = 1.21). The AUC of SM (d18:1/22:0) to identify hepatic steatosis in genotype 2 CHC was 0.726. Its sensitivity and negative predictive value reached 0.813 and 0.886, respectively. This study suggested that altered plasma SM (d18:1/22:0) was closely related to hepatic steatosis in chronic HCV infection, especially with genotype 2. Experimental studies are needed to determine further the underlying mechanisms responsible for these associations.


Similar content being viewed by others
References
Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G et al (2006) Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology 130:1636–1642
Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R (2002) Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol 37:837–842
Cross TJ, Quaglia A, Hughes S, Joshi D, Harrison PM (2009) The impact of hepatic steatosis on the natural history of chronic hepatitis C infection. J Viral Hepat 16:492–499
Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM et al (2003) Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut 52:288–292
Fartoux L, Chazouillères O, Wendum D, Poupon R, Serfaty L (2005) Impact of steatosis on progression of fibrosis in patients with mild hepatitis C. Hepatology 41:82–87
Kurosaki M, Hosokawa T, Matsunaga K, Hirayama I, Tanaka T, Sato M et al (2010) Hepatic steatosis in chronic hepatitis C is a significant risk factor for developing hepatocellular carcinoma independent of age, sex, obesity, fibrosis stage and response to interferon therapy. Hepatol Res 40:870–877
Harrison SA, Brunt EM, Qazi RA, Oliver DA, Neuschwander-Tetri BA, Di Bisceglie AM et al (2005) Effect of significant histologic steatosis or steatohepatitis on response to antiviral therapy in patients with chronic hepatitis C. Clin Gastroenterol Hepatol 3:604–609
Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S et al (2003) Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 38:75–85
Akuta N, Suzuki F, Tsubota A, Suzuki Y, Someya T, Kobayashi M et al (2002) Efficacy of interferon monotherapy to 394 consecutive naive cases infected with hepatitis C virus genotype 2a in Japan: therapy efficacy as consequence of tripartite interaction of viral, host and interferon treatment-related factors. J Hepatol 37:831–836
Akuta N, Suzuki F, Suzuki Y, Sezaki H, Hosaka T, Someya T et al (2005) Hepatocyte steatosis is an important predictor of response to interferon (IFN) monotherapy in Japanese patients infected with HCV genotype 2a: virological features of IFN-resistant cases with hepatocyte steatosis. J Med Virol 75:550–558
Itoh Y, Nishimura T, Yamaguchi K, Yokomizo C, Fujii H, Minami M et al (2011) Hepatic steatosis in chronic hepatitis C patients infected with genotype 2 is associated with insulin resistance, hepatic fibrosis and affects cumulative positivity of serum hepatitis C virus RNA in peginterferon and ribavirin combination therapy. Hepatol Res 41:1145–1152
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function. J Lipid Res 50(Suppl):S91–S96
Marí M, Fernández-Checa JC (2007) Sphingolipid signalling and liver diseases. Liver Int 27:440–450
Umehara T, Sudoh M, Yasui F, Matsuda C, Hayashi Y, Chayama K et al (2006) Serine palmitoyltransferase inhibitor suppresses HCV replication in a mouse model. Biochem Biophys Res Commun 346:67–73
Sakamoto H, Okamoto K, Aoki M, Kato H, Katsume A, Ohta A et al (2005) Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat Chem Biol 1:333–337
Katsume A, Tokunaga Y, Hirata Y, Munakata T, Saito M, Hayashi H et al (2013) A serine palmitoyltransferase inhibitor blocks hepatitis C virus replication in human hepatocytes. Gastroenterology 145:865–873
Bijl N, Sokolović M, Vrins C, Langeveld M, Moerland PD, Ottenhoff R et al (2009) Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 50:1431–1441
Zhao H, Przybylska M, Wu IH, Zhang J, Maniatis P, Pacheco J et al (2009) Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology 50:85–93
Qu F, Wu CS, Hou JF, Jin Y, Zhang JL (2012) Sphingolipids as new biomarkers for assessment of delayed-type hypersensitivity and response to triptolide. PLoS One 7:e52454
Qu F, Zheng SJ, Wu CS, Jia ZX, Zhang JL, Duan ZP (2014) Lipidomic profiling of plasma in patients with chronic hepatitis C infection. Anal Bioanal Chem 406:555–564
Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases (2009) Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 49:1335–1374
Hepatotogy Branch, Infectious and Parasitology Branch, Chinese Medical Association (2004) Guideline of prevention and treatment of hepatitis C. Zhonghua Yu Fang Yi Xue Za Zhi 38:210–215
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474
Brunt EM (2007) Pathology of fatty liver disease. Mod Pathol 20(Suppl 1):S40–S48
Cammà C, Bruno S, Di Marco V, Di Bona D, Rumi M, Vinci M et al (2006) Insulin resistance is associated with steatosis in nondiabetic patients with genotype 1 chronic hepatitis C. Hepatology 43:64–71
Asselah T, Rubbia-Brandt L, Marcellin P, Negro F (2006) Steatosis in chronic hepatitis C: why does it really matter? Gut 55:123–130
Rubbia-Brandt L, Quadri R, Abid K, Giostra E, Malé PJ, Mentha G et al (2000) Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol 33:106–115
Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G (2001) Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 33:1358–1364
Rubbia-Brandt L, Fabris P, Paganin S, Leandro G, Male PJ, Giostra E et al (2004) Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut 53:406–412
Deevska GM, Rozenova KA, Giltiay NV, Chambers MA, White J, Boyanovsky BB et al (2009) Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J Biol Chem 284:8359–8368
Cui Y, Jia J (2013) Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 28(Suppl 1):7–10
Acknowledgments
This work was funded by the National Science and Technology Key Project of China on “Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment” (2012ZX10002004-006, 2012ZX10004904-003-001, 2013ZX10002002-006); Ministry of Science and Technology of China (2012ZX09301002-006); The High Technical Personnel Training Item in Beijing Health System (2011-3-083); The Beijing Municipal Science & Technology Commission (no. Z131107002213019); The Special Scientific Research Fund for Beijing Health Development (2011-2018-04); YouAn Scientific Research Fund for Liver Disease and HIV/AIDS (BJYAH-2011-045).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jun-Feng Li, Feng Qu, and Su-Jun Zheng contributed equally to this work.
Zhong-Ping Duan and Jin-Lan Zhang contributed equally to this work and should be considered as co-corresponding authors.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Supplementary Table 1
(DOC 56 kb)
Supplementary Fig. 1
Relation between Cer (d18:1/24:0)-1-P and hepatic steatosis type in CHC. a The p-value (0.109) was acquired by one-way analysis of variance among three groups, and p-values between two groups were analyzed by the least significant difference t-test. b The p-value was acquired by the independent-samples t-test. c The p-value was acquired by the independent-samples t-test. (GIF 31 kb)
Supplementary Fig. 2
Relation between SM (d18:1/22:0) and hepatic steatosis type in CHC. a The p-value (0.287) was acquired by one-way analysis of variance among three groups, and p-values between two groups were analyzed by the least significant difference t-test. b The p-value was acquired by the independent-samples t-test. c The p-value was acquired by the independent-samples t-test. (GIF 31 kb)
Rights and permissions
About this article
Cite this article
Li, JF., Qu, F., Zheng, SJ. et al. Elevated plasma sphingomyelin (d18:1/22:0) is closely related to hepatic steatosis in patients with chronic hepatitis C virus infection. Eur J Clin Microbiol Infect Dis 33, 1725–1732 (2014). https://doi.org/10.1007/s10096-014-2123-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2123-x