Abstract
The frequency of Clostridium difficile infection (CDI)-related hospitalizations is increasing. The aim of this study was to determine the extent of CDI among children hospitalized with diarrhea, risk factors or predictors for severe CDI, the prevalence of NAP1, and to compare the course of CDI depending on bacteria toxicity profile. A retrospective analysis of case records of 64 children (age range 3 months–16 years, median age 2.12 years) with CDI as defined by diarrheal disease and positive polymerase chain reaction (PCR) test (Xpert C. difficile) was conducted. Modified national adult guidelines were used to assess the severity of CDI. CDIs represented 2.7 % of patients with diarrhea (13.5 cases per 1,000 admissions). Thirty-three CDIs (52 %) were community-associated. Antibacterial use preceded CDI in 61 patients (95 %). Seventeen cases (27 %) were binary toxin-positive (CDT+), 13 of which were NAP1 (20.5 %). Over 75 % of CDIs with NAP1 was hospital-acquired, and more often proceeded with generalized infection (p < 0.05). Risk factors for severe CDI (34 %) included NAP1 [odds ratio (OR), 4.85; 95 % confidence interval (Cl), 1.23, 21.86) and co-morbidities (OR, 4.25; 95 % Cl, 1.34, 14.38). Diarrhea ≥10 stools daily was associated with severe CDI (p = 0.01). Recurrence occurred in three patients (4.5 %). There was no mortality. C. difficile is an important factor of antibiotic-associated diarrhea in children. Co-morbidities and NAP1 predispose to severe CDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridium difficile (CD) was first described in 1935. The bacteria was found in stool specimens from healthy neonates, which led to its classification as a commensal [1], and was not first associated with disease until 1978 [2]. Currently, CD is one of the main factors of nosocomial infections that cause a spectrum of antibiotic-associated colitis (AAC), ranging from mild diarrhea to toxic megacolon [3–5]. Its role is growing as a result of the common use of broad-spectrum antibiotics, population aging, an increasing number of people afflicted by chronic conditions, and severe diseases requiring healthcare interventions [6–10]. It is estimated that, in the last decade, the rates of Clostridium difficile infection (CDI) have at least doubled [6, 11, 12]. The epidemiology of CDI in adults has changed significantly, including severe course of infections, high rate of relapse, deaths, and cases of community-acquired CDI without typical risk factors, including hospitalization and exposure to antibiotics [6, 13, 14]. These changes in adults have led us to consider a similar change in children. An important role in the changing epidemiology of CD has been the appearance of an epidemic hypervirulent strain, North American Pulsed Field Type 1, PCR ribotype 027 (NAP1), which has been responsible for outbreaks worldwide [15, 16]. The prevalence of NAP1 in adults varies widely, depending on the geographical region, although instances of 82 % prevalence have been reported [17, 18]. There are few studies which have reported on the prevalence of NAP1 in children ranging in age from 0 to 19 years [19, 20].
Despite the increasing number of CDIs and their severity [21, 22], CD continues to be an underestimated cause of diarrhea in patients <18 years of age. One reason for the underestimation of CDI in children is the high rate of asymptomatic colonization (in infants 14–70 %; in children 1–2 years of age, approximately 6 %), followed by a common perception that young children are not susceptible to CDI [23–25]. However, data indicate that this perception is only valid for neonates [26]. Neonates do occasionally develop CDI and the frequency of infection appears to have remained constant. The lack of susceptibility likely derives from the immaturity of neonate enterocytes and corresponding lack of toxin A receptors [27]. In all other groups of children, the number of CDIs and CDI-related hospitalizations (CDI-RH) continues to grow [26, 28]. A relatively large amount of data exists regarding prominent pediatric patients burdened with a high risk of CDI development, including Hirschsprung’s disease, inflammatory bowel disease, malignancies, hematological disorders, and immunodeficiency [29–35]. It has been shown that co-morbidities such as complex chronic conditions (CCCs) and severe underlying medical conditions might have increased the risk for C. difficile-associated disease (CDAD) due to the more frequent contact with healthcare facilities or exposure to antibiotics [9, 28]. Despite growing interest in CDAD in children, much less has been reported on the epidemiology and severity of CDI in the pediatric population.
The aim of this study was to assess the extent of CDI among children hospitalized with diarrhea, to assess the potential risk factors of CDI, to determine risk factors and predictors of severe CDI, to evaluate the rate of infections with NAP1, and to compare the course of the infection and the response to treatment with the bacteria toxicity profile.
Materials and methods
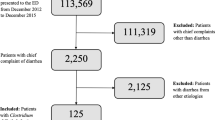
The study was conducted among children (<18 years of age) with CDI who were inpatients at the Hospital for Infectious Diseases in Bydgoszcz (HIDB) between December 12, 2010 and February 15, 2012. CDI cases were defined as patients with diarrhea and a positive Xpert C. difficile® polymerase chain reaction (PCR) test (sensitivity 95 %, specificity 100 %). The Xpert® C. difficile (Cepheid) PCR test identifies genes associated with CD in stool specimens: tcdB toxin gene, cdt binary toxin gene, and a deletion in the pathogenicity locus gene tcdC at nucleotide 117 present in NAP1 ribotype 027. Only patients presenting with their first episode were eligible for the study.
The distribution of diarrhea-associated hospitalizations by etiology and the rates of CD diarrheal diseases in patients were established. Additionally, the internal laboratory records for all CD-positive stools were reviewed.
To help assess the role of CDI in the pediatric population, patients were divided into seven age groups with potentially differing susceptibility to CDI. The age groups were: neonates, non-newborn infants, children >1–2, 3–4, 5–10, 11–15, and 16–18 years of age).
We performed a retrospective analysis of the clinical case records of children with CDI to collect demographic data, to evaluate the occurrence of potential risk factors for CDI, co-morbidities, toxicity profile of the strains, symptoms and the course of infection, as well as complications and outcomes. Follow-up phone calls to guardians were conducted in instances where case records were lacking information. Risk factors suspected for CDI included: age, prior antibiotic exposure (within 8 weeks before the CDI episode), hospitalization, recent use of acid-blocking agents (<4 weeks), and co-morbid conditions: severe diseases and CCCs (Table 1). To determine and qualify underlying chronic conditions, we used the classification system for pediatric CCCs, which includes nine categories of cancer and non-cancer conditions [36].
There is no accepted definition of severe CDI in children. Thus, this study employed criteria for severe and severe-complicated CDI in adults [37, 38], with some modifications based upon the criteria of previously reported pediatric studies [21, 39].
Severe disease was defined by two or more of the following: fever ≥38.5 ºC, white blood cell count (WBC) ≥15,000/mm3, elevated age-adjusted serum creatinine, albumin <2.5 g/dl. CDI was classified as severe-complicated if the patient had at least one of following complications: hypotension, sepsis, ileus, pseudomembranous colitis, toxic megacolon, gastrointestinal perforation, the need for intensive care unit admission, surgery for a CDI-related complication, or death. To assess the correlation between CDI severity and other potential risk factors or predictors, we calculated p-values for additional data (Table 2).
Statistical analysis
The incidence of CDI cases in HIDB was calculated per 1,000 admissions. The summary statistics for normally distributed continuous variables were expressed as the mean and standard deviation and as the median with interquartile range (25th and 75th percentiles) for non-normally distributed variables. Categorical variables were expressed as frequencies. Differences between continuous normally distributed variables were analyzed by the t-test for normally distributed data or by the Mann–Whitney U-test for non-normally distributed data. Differences for categorical variables were tested using the Chi-square or Fisher’s exact test for independence.
The assessment of the potential risk factors of severe CDI was carried out by the use of univariable and multivariable logistic regression. The risk factors with p-values smaller than 0.2 in the univariable analysis were considered for inclusion into the multivariable model. A cut-off of p < 0.05 was used to determine statistical significance. The software package R version 2.10.1 was used for the analysis.
Definitions:
- CDI:
-
Presence of diarrhea (≥3 liquid stools within 24 h) and positive Xpert C. difficile PCR test.
- Hospital-acquired CDAD (HA-CDAD):
-
Symptoms occurred over 48 h after current admission or CDI diagnosed within 48 h of readmission in patients hospitalized in the previous four weeks.
- Community-associated CDAD (CA-CDAD):
-
Symptoms occurred before or within 48 h of the current admission and over 12 weeks after previous discharge.
- Indeterminate CDAD:
-
Symptoms occurred outside hospital between >4–12 weeks after discharge.
- Antibiotic-associated diarrhea (AAD):
-
Unexplained diarrhea occurring between 2 h to 2 months after starting antibiotics.
- Relapse of CDI:
-
Recurrence of diarrhea within 2 to 8 weeks of a previous CDI episode. Recurrence of diarrhea <2 weeks from the previous episode was considered to be a continuation of the previous episode.
- Re-infection of CDI:
-
Recurrence of diarrhea over 8 weeks after a previous CDI episode.
Results
Study population
During the 26-month observation period, 64 children (33 males, 31 females) ranging in age from 3 months to 16 years were diagnosed with CDI. The mean age of the patients was 3.33 years (median 2.12 years). The age groups 1–2 and 3–4 years accounted for the largest proportion of all CDI-RH (30 and 28 %, respectively); however, these groups had the second and third highest incidences CDI-RH of all age groups (3.8 and 2.9 %, respectively). The highest rate was revealed among non-newborn infants (4.8 %), but this group accounted for the lowest rate of all CDI-RH (14 %) (Fig. 1a). There were no CDI cases among neonates. This group constituted the lowest proportion of all hospitalized children (1.35 %).
CDI cases represented 2.8 % of 2,238 children hospitalized due to diarrhea (Fig. 1b). It constituted 13.5 cases per 1,000 pediatric admissions (64/4,656). CD comprised the fourth highest cause of diarrhea in hospitalized children (Fig. 2). The majority of the patients with CDI (83 %) were simultaneously tested for other common agents of gastrointestinal infections (rotavirus, enteric adenovirus, bacterial agents including Campylobacter spp.). Co-infection was revealed in 12 children (19 %): with rotavirus (n = 8), Salmonella (n = 2), Yersinia (n = 2), adenovirus (n = 1), norovirus (n = 1), and Campylobacter (n = 1). In three of these cases, rotaviral diarrhea followed CDI as a nosocomial infection. In three patients, the co-infection of three pathogens was detected.
In 47 cases (73 %), toxin B-producing strains (B+) were revealed, while in the remaining 17 (27 %), strains producing toxin B and binary toxin (B+/CDT+) were observed. Thirteen of them exhibited the single nucleotide deletion at position 117 typical of hiperwirulent strains NAP1/027 (Fig. 3). The median age of children with NAP1 was 2.58 years (mean 3.74 years). The majority of cases with NAP1 (69 %) were classified as hospital-acquired (p = 0.02).
Among clinical symptoms, bloody diarrhea occurred in 37.5 % of the patients, abdominal pain and cramping were listed in 69 % of the children, and over 60 % of CDI cases conducted with fever (>38 ºC). Three-fourths of the patients had common complications of diarrhea: dehydration and electrolytes disturbances. Twenty-eight patients had abnormalities on abdominal sonography (44 %): enlarged mesenteric lymph nodes in 20 patients, five cases with free fluid in the peritoneal space, and features of colitis in three cases. Pseudomembranous colitis was confirmed by endoscopy in one patient with congenital immunodeficiency syndrome. Other parents did not give permission to carry out endoscopy procedures. One patient (1.5 %) burdened with CCCs (short bowel syndrome, uro- and ileostomy) needed surgical management due to intussusception and ileus.
Over half of the children (36/64) were treated with single orally administered metronidazole and eight children received both vancomycin (orally) and metronidazole (orally or intravenous) concurrently during hospitalization. Approximately 9 % (6/64) received single vancomycin (orally) and three of them continued the treatment with metronidazole (orally) after discharge. About 20 % of the patients (13/64) did not require treatment because of self-limitation of the diarrhea course after antibiotic therapy discontinuation: nine patients with B+, three with non-NAP1, CDT+, and one case with NAP1. One child was treated with rifaximin on account of Salmonella co-infection.
Risk factors for CDI
In 61 patients (95 %), CDI was preceded by antibiotics administration. Forty-nine (80 %) patients developed CDI during antibiotic therapy, lasting 2–30 days (mean 7.0 days; median 5.5 days) or 2 days to 6 weeks after the end of the treatment lasting, on average, 7.8 days (12/61, 20 %). Almost 40 % of the patients underwent intensive antibiotic therapy with two to four antibiotics (in polypragmasy or sequentially) and/or long-lasting treatment over 2 weeks. The most often administered antibiotics were second- and third-generation cephalosporins (41/60, 68 %) and amoxicillin/clavulanic acid (25/60, 42 %) administered i.v., orally, and/or i.m. (Fig. 4). There was no correlation between CDI and antibiotic therapy in three patients (5 %), all bottle-fed neonates who had never received antibiotics before, and their CDI was judged to be community-acquired.
Exposure to antibiotics during the 6 weeks preceding CDI. The numbers and percentages do not sum to the total number of patients with diarrhea or 100 % because some children had multiple antibiotics. (AM-CL amoxicillin/clavulanic acid, Amox amoxicillin, Ceph III third-generation cephalosporin, Ceph II second-generation cephalosporin, AMK amikacin, Clarithro clarithromycin, TMP-SMX trimethoprim–sulfamethoxazole, Clinda clindamycin, MER meropenem)
Among 33 cases (52 %) classified as CA-CDAD, the majority (30/33) had used antibiotics previously due to common infections of the respiratory tract, otitis media, gingivitis, and gastroenteritis (median age 3.8 years). Correlation between hospitalization and CDI was found in 27 (42 %) children (median age 1.8 years). Four cases were undefined.
The presence of co-morbidities was revealed in 22 (34 %) patients. Severe, life-threatening disease preceded the development of CDI in 17 children and CCCs in 15 cases (Table 1). Ten patients (15 %) received proton-pump inhibitors (PPIs) prior to the onset of CDI.
Treatment response and outcomes
Good response to the treatment was observed in almost all patients with normalization of the stools frequency and consistency and regression of clinical symptoms after an average of 3–4 days following the treatment. Ineffectiveness of the first-line therapy (diarrhea >6 days despite the treatment) occurred in two cases: one treated with metronidazole (B+/CDT−) and the other treated with vancomycin (NAP1). In both cases, the second-line treatment (concurrent administration of metronidazole and vancomycin) was effective. Recurrence of CDI symptoms was observed in 3 patients (4.5 %): relapse in one case (1.5 %) with NAP1 burdened with several factors, and in two cases (B+) re-infection occurred (3 %). There were no deaths.
Risk factors for severe disease
Twenty-two patients (34 %) met the criteria for severe or severe-complicated CDI: nine cases with NAP1, 12 with B+, and one patient with non-NAP1, CDT+. CDI with NAP1 significantly more frequently (53 %) proceeded with generalized infection (range of plasma procalcitonin levels: 0.99–39 ng/ml and CRP values: 15–procalcitonin occurred in patients with co-morbidities (Table 2). In comparison, CDI caused by non-NAP B+/CDT+ and B+/CDT− strains was associated with septicemia in 25 % (1/4) and 8.5 % (4/47) of the cases, respectively. There were no significant differences in the course of CDI caused by NAP1 and non-NAP1 considering the period of diarrhea and type of stools, fever, WBC, and the response to the treatment (Table 2). CDI associated with ≥10 stools per day was significantly more frequent for severe CDI (p = 0.01).
Logistic regression analysis was used to assess the potential risk factors for severe CDI (Table 3). Ribotype NAP1 027 and co-morbidities were associated with severe CDI in both the univariate and in the multivariate analyses. Patients with NAP1 027 were over four times more likely to develop severe CDI than patients with non-NAP1 [odds ratio (OR) = 4.85 ; 95 % confidence interval (CI) 1.23, 21.86]. Patients with co-morbidities were also over four times more likely to develop severe CDI than patients who did not have any co-morbidities (OR = 4.25; 95 % CI 1.34, 14.38).
Discussion
The results of this study indicate that CD is a significant cause of diarrhea and CDI-RH in children. The frequency of CDI for pediatric admissions observed in this study (13.5/1,000) is relatively high compared to other studies. The rate of CDI-RH was assessed from admissions to HIDB, which is the largest reference hospital for infectious diseases in the Kuyavian-Pomeranian Province and receives pediatric patients from the surrounding regions. The Department of Pediatric Infectious Diseases and Hepatology receives patients after treatment failure at other institutions, including outpatient clinics and hospitals. A considerable number of the patients include children with poorly treatable diarrhea. Thus, a higher frequency of CDI hospitalizations compared to previous studies is not surprising.
Despite the lower incidence of CDI in previous studies, most have suggested the continued increase of CDI-RH in pediatric patients. Kim et al. demonstrated that the annual incidence of CDAD increased between 2001 and 2006, from 2.6 to 4.0/1,000 admissions. The study was conducted at 22 US children’s hospitals. One of these hospitals reported an increase from 3.6 to 11.8 cases of CDI per 1,000 admissions, a 227 % increase [28]. Similar trends were found by Zilberberg et al. in a US cross-sectional study. The rate of CDI-RH increased from 7.24 to 12.8/10,000 admissions between 1997 and 2006 [26]. The average annual percentage increase of CDI-RH in studies by Zilberberg et al. and Cade et al. totaled 9 % and 14.9 %, respectively [26, 40]. Similar observations involve CA-CDAD. Baker et al., in a 3-year study of children with non-specific gastrointestinal complaints who underwent colonoscopy, had noted that the proportion of CD-positive children increased from 9.5 % (2006) to 27 % (2008) [41]. A study by Benson et al. revealed a steady increase in the number of patients with CDAD in the outpatient setting (1.18 cases/1,000 in 2001 vs. 2.47 cases/1,000 in 2006) and they also noted a significant decrease of CDI among the outpatients [13].
Routine testing for CD is discouraged in children <2 years of age [12]. Yet, this group of patients constituted over 43 % (28/64) of the children with CDI hospitalized at HIDB. It is estimated that most CDI cases in infants represents colonization; however, in our study, infants had a higher rate of CDI. It is impossible to definitively differentiate between symptomatic disease or colonization in these cases, but rapid response to treatment led us to assume that the latter had occurred. The highest incidence of CDI was for non-newborns infants. They constituted 4.2 % among non-newborn infants with gastroenteritis and 2.9 % of all hospitalized children in this age group. However, in relation to all patients with CDI, this group accounted for only 14 % (9/64). Children in the age groups ≥1–2 and 3–4 years represented the highest proportion of all CDI cases (30 and 28 %, respectively) and had the second and third highest rates in these age groups. The lack of CDI in newborns confirms that this group of children is not susceptible to CD and testing due to CDI should be restricted to newborns burdened with risk factors. Another possible reason for why this age group lacks CDI is that most newborns with diarrhea are hospitalized in neonatal units. These results are partially consistent with findings from previous studies. In a US study by Zilberberg et al., newborns had the lowest annual CDI rate of all pediatric age groups (0.5/10,000) [26]. The highest incidence of CDI-RH was observed in children in the age group 1–4 years (44.9/10,000), but non-newborn infants had third highest rate, after children 5–9 years of age. Unlike our observations, in the study by Zilberberg et al., non-newborn infants consistently accounted for the largest proportion of all pediatric CDI-RH cases during the observation period from 1997 to 2006 [26]. In the study by Kim et al., neonates represented 5 % and non-newborn infants represented 26 %. They noted an increasing frequency of CDI over the 5-year study period in children in age groups 1–5 years and 5–17 years, but did not observe such trends in children <1 year of age [21]. The median age of the patients was 4 years. A study by Morinville and McDonald including 200 children reported a median age of 2.6 years and a mean age of 5.4 years, which is similar to our study (median and mean age 2.12 and 3.33 years, respectively) [42].
Antibiotic therapy remains the main factor for developing CDI in children, particularly long-term or polyantibiotic treatment [43, 44]. It preceded CDI in our study in over 94 % of the cases. There was no correlation between antimicrobial treatment and CDI in nearly 6 % of cases (three infants who had never used antibiotics previously). The majority of patients presenting CDI symptoms were concurrently undergoing treatment with antibiotics known to bring about an increased risk of CDI (mostly the broad-spectrum cephalosporins and penicillins). The high correlation of CDI with antibacterial use was determined by having full access to medical documentation, including phone contact with guardians as needed. A higher proportion of CDI linked to antibiotic use (100 %) was found in a study by Oğuz et al., particularly with third-generation cephalosporins and ampicillin–sulbactam with aminoglycoside [45]. In other studies, the correlation with prior antibiotic therapy was lower. The investigations by Benson et al. and Morinville and McDonald revealed documented exposure to antibiotics in 57 and 74 % of patients with CDI, respectively [13, 42]. The study by Morinville and McDonald reported that cephalosporins were more often employed and one-third of CDI cases underwent polyantibiotic therapy [42]. In the study by Kim et al., 62 % of patients had had recent antibiotic exposure [21].
In accordance with changes observed in the epidemiology of CDI in our cohort, CA-CDAD cases predominated and accounted for 52 % of the patients. The higher incidence of CA-CDAD cases in pediatric residents revealed by Khanna et al. constituted 75 % [46].
PPIs were used by 15 % of our patients, but were not a significant factor for CDI. Baker et al. detected that significantly more CD-positive pediatric patients had used H2 receptor antagonists within the month before testing than CD-negative patients, but they did not find differences with respect to PPI use [41].
In over half of the children, we found two to four CDI risk factors. There were no typical risk factors in only three infants with CA-CDAD (6 %). A similar percentage of CDIs with no typical risk factors (6.5 %) was reported by Morinville and McDonald [42].
There is a lack of data concerning the prevalence of NAP1 in the pediatric population. The prevalence of NAP1 found in our study (20.5 %) is similar to that in the study conducted by Toltzis et al. in children with gastrointestinal symptoms. They found NAP1 in 19 % of samples and non-NAP (CDT+) in 1.5 % of isolates (vs. 6.5 % in our study) [20]. In the prospective study by Kim et al., NAP1 was found in 11 % of CDI cases [21]. In contrast, Stoesser et al. compared CD strains isolated from children with that circulating in adults and did not find any isolates of NAP1 in healthy children or those with diarrhea [19].
Consistent with findings from recent studies in adults, CDI caused by NAP1 was associated with the most severe disease. NAP1 cases constituted 41 % of severe CDIs and over half proceeded with generalized infection. CDIs with NAP1 were usually hospital-acquired. This is probably the result of co-morbidities in these patients (p < 0.05) increasing the amount of contact with healthcare settings, antibiotic therapies, and infection carriage. Thus, NAP1 and co-morbidities are significant risk factors for severe CDI. Profuse diarrhea ≥10 stools per day was a single predictor associated with severe CDI (p = 0.01). Kim et al. reported no association between disease severity and NAP1, although co-morbidity, age, and treatment with three or more antibiotics classes were all associated with severe CDI [21]. In another study by Kim et al., underlying chronic conditions were present in 67 % of children with CDI [28].
CDI in children is suspected to be less frequent and less severe than in adults. In comparison to previous pediatric studies, the rate of recurrences was considerably lower (about 4.5 %) and there were no deaths. There were no differences in the response to the treatment between CDI caused by NAP and non-NAP strains. Similar findings were presented by Pai et al. in their study over a 5-year period. They observed low morbidity and no CD-related deaths [39]. Khanna et al. reported treatment failure in 18 % of patients treated with metronidazole, but none among those treated with vancomycin [46]. In the study by Kim et al., relapse concerned one-fourth of CDIs, CDI-related complications occurred in 17 %, over half of the patients with CDI had severe disease, and there were 2 % deaths [21]. In the study by Morinville and McDonald, the mortality was lower (1 %), but relapses accounted for over 30 %, mostly in patients with immunodeficiency [42], which was approximate to that reported among adults [47].
Conclusion
Clostridium difficile (CD) is an important etiological factor of antibiotic-associated diarrhea (AAD), especially in children hospitalized due to severe illnesses or burdened with complex chronic conditions (CCCs), as well as community-associated C. difficile-associated disease (CA-CDAD). Non-newborn infants and children below 2 years of age are at risk of symptomatic C. difficile infection (CDI) and constitute a significant percentage of children hospitalized due to CDI. Antibiotic therapy is the main risk factor of CDI in children. NAP1 strain CDI is more likely to present a serious course of the disease and proceed with generalized infection. NAP1 and co-morbidities constitute risk factors for severe CDI. CD strains cause less significant disease among children than adults, as judged by the number of relapses, poor outcomes, and deaths.
References
Hall IC, O’Toole E (1935) Intestinal flora in new-born infants: with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 49:390–402
Larson HE, Price AB, Honour P, Borriello SP (1978) Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet 1:1063–1066
McFarland LV (2008) Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol 5:40–48
Elliott B, Chang BJ, Golledge CL, Riley TV (2007) Clostridium difficile-associated diarrhoea. Intern Med J 37:561–568
Karas JA, Enoch DA, Aliyu SH (2010) A review of mortality due to Clostridium difficile infection. J Infect 61:1–8
Zilberberg MD, Shorr AF, Kollef MH (2008) Increase in adult Clostridium difficile-related hospitalizations and case–fatality rate, United States, 2000–2005. Emerg Infect Dis 14:929–931
Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN (2007) Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg 142:624–631, discussion 631
Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE; SHEA Long-Term-Care Committee (2002) Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol 23:696–703
Kyne L, Sougioultzis S, McFarland LV, Kelly CP (2002) Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 23:653–659
McFarland LV, Surawicz CM, Stamm WE (1990) Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis 162:678–684
Zilberberg MD, Shorr AF, Kollef MH (2008) Increase in Clostridium difficile-related hospitalizations among infants in the United States, 2000–2005. Pediatr Infect Dis J 27:1111–1113
Sharland MC, Cant A, Shingadia D (2011) Clostridium difficile infection. In: Manual of childhood infections: the blue book. Oxford, pp 487–495
Benson L, Song X, Campos J, Singh N (2007) Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 28:1233–1235
Frost F, Craun GF, Calderon RL (1998) Increasing hospitalization and death possibly due to Clostridium difficile diarrheal disease. Emerg Infect Dis 4:619–625
O’Connor JR, Johnson S, Gerding DN (2009) Clostridium difficile infection caused by the epidemic B1/NAP1/027 strain. Gastroenterology 136:1913–1924
Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J et al (2005) Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084
Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S et al (2005) A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 353:2442–2449
MacCannell DR, Louie TJ, Gregson DB, Laverdiere M, Labbe AC, Laing F et al (2006) Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J Clin Microbiol 44:2147–2152
Stoesser N, Crook DW, Fung R, Griffiths D, Harding RM, Kachrimanidou M et al (2011) Molecular epidemiology of Clostridium difficile strains in children compared with that of strains circulating in adults with Clostridium difficile-associated infection. J Clin Microbiol 49:3994–3996
Toltzis P, Kim J, Dul M, Zoltanski J, Smathers S, Zaoutis T (2009) Presence of the epidemic North American Pulsed Field type 1 Clostridium difficile strain in hospitalized children. J Pediatr 154:607–608
Kim J, Shaklee JF, Smathers S, Prasad P, Asti L, Zoltanski J et al (2012) Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J 31:134–138
Pokorn M, Radsel A, Cizman M, Jereb M, Karner P, Kalan G et al (2008) Severe Clostridium difficile-associated disease in children. Pediatr Infect Dis J 27:944–946
Al-Jumaili IJ, Shibley M, Lishman AH, Record CO (1984) Incidence and origin of Clostridium difficile in neonates. J Clin Microbiol 19:77–78
Emeruwa AC, Oguike JU (1990) Incidence of cytotoxin producing isolates of Clostridium difficile in faeces of neonates and children in Nigeria. Microbiologica 13:323–328
Wultańska D, Obuch-Woszczatyński P, Banaszkiewicz A, Radzikowski A, Pituch H, Młynarczyk G (2010) Prevalence of Clostridium difficile in the gastrointestinal tract of hospitalized children under two years of age. Med Dosw Mikrobiol 62:77–84
Zilberberg MD, Tillotson GS, McDonald C (2010) Clostridium difficile infections among hospitalized children, United States, 1997–2006. Emerg Infect Dis 16:604–609
Eglow R, Pothoulakis C, Itzkowitz S, Israel EJ, O’Keane CJ, Gong D et al (1992) Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest 90:822–829
Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T (2008) Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics 122:1266–1270
Qualman SJ, Petric M, Karmali MA, Smith CR, Hamilton SR (1990) Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am J Clin Pathol 94:410–416
Simon A, Fleischhack G, Hasan C, Bode U, Engelhart S, Kramer MH (2000) Surveillance for nosocomial and central line-related infections among pediatric hematology–oncology patients. Infect Control Hosp Epidemiol 21:592–596
Burgner D, Siarakas S, Eagles G, McCarthy A, Bradbury R, Stevens M (1997) A prospective study of Clostridium difficile infection and colonization in pediatric oncology patients. Pediatr Infect Dis J 16:1131–1134
Brunetto AL, Pearson AD, Craft AW, Pedler SJ (1988) Clostridium difficile in an oncology unit. Arch Dis Child 63:979–981
Thomas DF, Fernie DS, Bayston R, Spitz L, Nixon HH (1986) Enterocolitis in Hirschsprung’s disease: a controlled study of the etiologic role of Clostridium difficile. J Pediatr Surg 21:22–25
Hardy SP, Bayston R, Spitz L (1993) Prolonged carriage of Clostridium difficile in Hirschsprung’s disease. Arch Dis Child 69:221–224
Wultańska D, Banaszkiewicz A, Radzikowski A, Obuch-Woszczatyński P, Młynarczyk G, Brazier JS et al (2010) Clostridium difficile infection in Polish pediatric outpatients with inflammatory bowel disease. Eur J Clin Microbiol Infect Dis 29:1265–1270
Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD (2001) Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics 107:E99
Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC et al (2010) Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455
Hryniewicz W, Martirosian G, Ozorowski T (2011) Zakażenia Clostridium difficile: diagnostyka, terapia, profilaktyka. Narodowy program ochrony antybiotyków, Warszawa
Pai S, Aliyu SH, Enoch DA, Karas JA (2012) Five years experience of Clostridium difficile infection in children at a UK tertiary hospital: proposed criteria for diagnosis and management. PLoS One 7:e51728
Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB (2011) Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med 165:451–457
Baker SS, Faden H, Sayej W, Patel R, Baker RD (2010) Increasing incidence of community-associated atypical Clostridium difficile disease in children. Clin Pediatr (Phila) 49:644–647
Morinville V, McDonald J (2005) Clostridium difficile-associated diarrhea in 200 Canadian children. Can J Gastroenterol 19:497–501
Bignardi GE (1998) Risk factors for Clostridium difficile infection. J Hosp Infect 40:1–15
Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA (2008) Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis 46(Suppl 1):S19–S31
Oğuz F, Uysal G, Daşdemir S, Oskovi H, Vidinlisan S (2001) The role of Clostridium difficile in childhood nosocomial diarrhea. Scand J Infect Dis 33:731–733
Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL et al (2012) The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol 107:89–95
Johnson S (2009) Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect 58:403–410
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Dulęba, K., Pawłowska, M. & Wietlicka-Piszcz, M. Clostridium difficile infection in children hospitalized due to diarrhea. Eur J Clin Microbiol Infect Dis 33, 201–209 (2014). https://doi.org/10.1007/s10096-013-1946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1946-1