Abstract
The purpose of this investigation was to characterize the management and prognosis of severe Pneumocystis jirovecii pneumonia (PJP) in human immunodeficiency virus (HIV)-negative patients. An observational cohort study of HIV-negative adults with PJP documented by bronchoalveolar lavage (BAL) through Gomori–Grocott staining or immunofluorescence, admitted to one intensive care unit (ICU) for acute respiratory failure, was undertaken. From 1990 to 2010, 70 patients (24 females, 46 males) were included, with a mean age of 58.6 ± 18.3 years. The mean Simplified Acute Physiology Score (SAPS)-II was 36.9 ± 20.4. Underlying conditions included hematologic malignancies (n = 21), vasculitis (n = 13), and solid tumors (n = 13). Most patients were receiving systemic corticosteroids (n = 63) and cytotoxic drugs (n = 51). Not a single patient received trimethoprim–sulfamethoxazole as PJP prophylaxis. Endotracheal intubation (ETI) was required in 42 patients (60.0 %), including 38 with acute respiratory distress syndrome (ARDS). In-ICU mortality was 52.9 % overall, reaching 80.9 % and 86.8 %, respectively, for patients who required ETI and for patients with ARDS. In the univariate analysis, in-ICU mortality was associated with SAPS-II (p = 0.0131), ARDS (p < 0.0001), shock (p < 0.0001), and herpes simplex virus (HSV) or cytomegalovirus (CMV) on BAL (p = 0.0031). In the multivariate analysis, only ARDS was associated with in-ICU mortality (odds ratio [OR] 23.4 [4.5–121.9], p < 0.0001). PJP in non-HIV patients remains a serious disease with high in-hospital mortality. Pulmonary co-infection with HSV or CMV may contribute to fatal outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1980s, most cases of Pneumocystis jirovecii pneumonia (PJP) have been documented in human immunodeficiency virus (HIV)-infected patients. In early series of acquired immunodeficiency syndrome (AIDS)-related PJP, the in-hospital mortality of patients who required mechanical ventilation during the course of PJP was 87 % [1]. With the advent of improved diagnostic methods for P. jirovecii detection, the introduction of adjunctive corticosteroids for the treatment of moderate to severe PJP [2–4], and progresses in supportive care for acute respiratory failure (ARF) in the intensive care units (ICUs), only 7.3 % of HIV-infected patients with PJP require endotracheal intubation (ETI) nowadays, and the in-hospital mortality rate of intubated patients decreased to 56.2 % [5, 6].
Since the late 1990s, the advent of combined antiretroviral therapy has dramatically reduced the incidence of HIV-associated PJP [7]. In parallel, following the increased use of immunosuppressive therapy for neoplastic diseases, inflammatory diseases, or solid organ transplant, the proportion of PJP diagnosed in non-HIV-infected patients increased [8–10]. In one series of PJP in non-HIV-infected patients, ETI was necessary in 65.6 % of cases, with an in-hospital mortality rate of 59.1 % [5]. This study, and others [11], suggested that PJP in non-HIV-infected patients may be more severe than PJP in HIV-infected patients, although limited data are available regarding the clinical course and prognosis factors of PJP in non-HIV-infected patients. Several investigators have suggested that advances in the management of HIV-associated PJP may be applied to PJP care in non-HIV-infected patients, including the use of adjunctive corticosteroids [12–14]. We aimed to determine the outcome of PJP in non-HIV-infected patients and the risk factors for death in this population.
Materials and methods
We performed a retrospective analysis of patients with microbiologically confirmed PJP who were admitted to our medical ICU for ARF. From January 1990 to June 2010, all cases of PJP documented by direct examination (Gomori–Grocott staining and/or immunofluorescence) on bronchoalveolar lavage (BAL), in patients who tested negative for HIV, were included. PJP documented only by P. jirovecii polymerase chain reaction (PCR) were not included, due to the substantial risk of false-positivity associated with this test [15]. We also excluded patients admitted to the ICU without ARF. Patients were identified through our department’s computerized database and through the mycology laboratory database. Data extracted included the following: demographics, Simplified Acute Physiology Score (SAPS)-II, ICU length of stay, outcome, delay between BAL and administration of the first agent active on P. jirovecii, co-infections, underlying immunosuppression, corticosteroid use before PJP diagnosis, PJP prophylaxis, antimicrobial agents, and corticosteroids use after PJP diagnosis. Viral tests are routinely performed in BAL fluid from immunocompromised patients in our institution. Over the study period (1990–2010), viral tests could include: (1) immunofluorescence (IF) with a panel of monoclonal antibodies against respiratory syncytial virus (RSV), A–B influenza viruses, 1–3 parainfluenza viruses, adenovirus, and herpes simplex viruses (HSV)-1 and -2; (2) inoculation onto MRC-5 and LLC-MK2 monolayer cells; (3) immunocytochemistry (ICC) directed against cytomegalovirus (CMV); (4) detection of HSV-1, HSV-2, CMV, varicella-zoster virus (VZV), Epstein–Barr virus (EBV), and human herpes virus (HHV)-6 genomes using a commercial method (Herpes Consensus®, Argène, France) or in-house PCR. Acute respiratory distress syndrome (ARDS) was defined as ARF with bilateral pulmonary infiltrates and PaO2:FiO2 ratio <200 mmHg (26.7 kPa), unrelated to left ventricular failure. Statistical analysis was done with SAS 9.2 (SAS Institute, Cary, NC, USA). Continuous variables were presented as mean ± standard deviation or median [interquartile range]. Categorical variables were expressed as percentages. Continuous variables were compared between groups using non-parametric Mann–Whitney U-tests, and categorical variables were compared using the Chi-square test or Fisher’s exact test. The variables included in the multivariate analysis were those with p < 0.1 in the univariate analysis. A p-value <0.05 was considered to be statistically significant.
Results
Demographic features
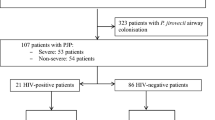
Between January 1990 and June 2010, 116 patients with PJP were admitted to our ICU. Of those, 39 HIV-infected patients were excluded, three patients were excluded because PJP was documented only by PCR, and four patients were excluded because they were not admitted to the ICU because of ARF (i.e., status epilepticus, n = 2; post-surgical care of solid organ transplant, n = 2). The final cohort consisted of 70 non-HIV-infected patients with documented PJP admitted to the ICU because of ARF. Baseline characteristics are detailed in Table 1.
Underlying immunosuppression and pneumocystosis prophylaxis
All patients with PJP were immunocompromised, and most of them (90.0 %) were receiving corticosteroids when PJP was diagnosed, with a mean daily prednisone-equivalent dose of 46.8 mg ± 138.1 on admission, associated in 72.9 % of cases with other immunosuppressive agents, including cyclophosphamide (n = 19), calcineurin inhibitor (n = 9), the combination of cyclophosphamide, adriamycine, and vincristine (n = 7), and methotrexate (n = 6). Fourteen patients were receiving only corticosteroids, with a mean daily prednisone-equivalent dose of 30.8 mg ± 26.6 on admission, as a prolonged treatment (>3 weeks) of various diseases: interstitial lung disease, n = 2; sarcoidosis, glomerulonephritis, polyarteritis nodosa, giant-cell arteritis, polymyalgia rheumatica, polyneuritis, acute alcoholic hepatitis, polymyositis, meningioma, post-radiation myelitis, pharyngeal carcinoma, and acute cophosis—one patient each. Only two patients were on PJP prophylaxis before PJP diagnosis, both with monthly aerosolized pentamidine. Of note, no patient developed PJP while on trimethoprim–sulfamethoxazole prophylaxis.
Pneumocystosis management in the ICU
BAL was performed in the ICU for most patients (n = 45), with a mean delay of 0.4 ± 5.1 days between ICU admission and BAL. PJP treatment was initiated before BAL or on the same day in most patients (n = 54). Only two patients were treated without trimethoprim–sulfamethoxazole, because of previously documented severe allergy: they both received intravenous and aerosolized pentamidine. In 11 patients (15.7 %), trimethoprim–sulfamethoxazole had to be discontinued before the planned duration of treatment because of allergy and/or cytopenia. Patients were then switched to pentamidine (n = 9) or atovaquone (n = 2). Data on corticosteroids use after PJP diagnosis were available for 51 patients. Of these, 43 (84.3 %) received corticosteroids: methylprednisolone (n = 39) or hemisuccinate hydrocortisone (n = 4). ETI was required because of ARF in 42 patients (60.0 %). The mean delay between ICU admission and ETI was 2.4 ± 6.1 days, and the mean duration of mechanical ventilation was 13.1 ± 11.4 days. PaO2:FiO2 was <200 in 38 patients (54.3 %). Non-invasive positive pressure ventilation was used in four patients (5.7 %). Extracorporeal membrane oxygenation (ECMO) was used for one patient with uncontrollable ARDS, who eventually died.
Outcome
Overall, 37 patients (52.9 %) died in the ICU. The mortality rate was 80.9 % among the 42 patients who required ETI and 86.8 % among the 38 patients who fulfilled criteria for ARDS. Twenty patients (29.8 %) required hemodialysis and 37 patients (55.2 %) received hemodynamic support with norepinephrine or epinephrine. Twenty patients (28.6 %) developed ventilator-associated pneumonia, of which ten were related to Pseudomonas aeruginosa. Other pathogens isolated in BAL are detailed in Table 2. The 15 patients (21.4 %) in whom HSV-1 and/or CMV were identified on BAL were treated with ganciclovir (n = 8) or acyclovir (n = 7). In the univariate analysis (Table 3), factors associated with death were SAPS-II (p < 0.0131), ARDS (p < 0.0001), shock (p < 0.0001), and BAL positive for HSV or CMV (p = 0.0031). In the multivariate analysis (Table 4), the only factor independently associated with death was ARDS (odds ratio [OR] 23.4 [4.5–121.9], p < 0.0001).
Discussion
To our knowledge, this is the largest cohort of PJP in non-HIV-infected patients admitted to the ICU. The high in-ICU mortality rate (i.e., 52.9 %) is similar to the 48 % reported by Monnet et al. in a cohort of 27 non-HIV-infected patients with PJP admitted to the ICU during the years 1993–2006, with similar severity scores (mean SAPS-II = 38, as compared to 36.9 in our cohort) [11]. Likewise, Mansharamani et al. observed a mortality rate of 59 % in a cohort of 33 non-HIV-infected patients admitted to the ICU during the years 1985–1995 [5], and the in-ICU mortality was 44.9 % in Delclaux et al.’s cohort of 31 non-HIV-infected patients with PJP during the years 1988–1996 [14]. Even studies not restricted to ICU patients have documented that PJP in non-HIV-infected patients is a life-threatening condition, with in-hospital mortality rates ranging from 35 to 50 % [16–18].
This high mortality rate must be kept in mind when considering PJP prophylaxis in patients with non-HIV-related severe immunosuppression: failure to protect ‘at-risk’ patients will carry a significant amount of fatalities. This is especially relevant given that daily or thrice-weekly trimethoprim–sulfamethoxazole has an efficacy rate approaching 100 % in the prevention of PJP [19]. Indeed, in our series as in others, no case of PJP was documented in patients receiving this prophylactic regimen. The American Thoracic Society (ATS) recommends PJP prophylaxis in patients with hematologic and solid malignancies receiving cytotoxic chemotherapies, organ transplantation, and those treated with immune-suppressive regimens for inflammatory conditions. In addition, the ATS advises to consider prophylaxis during time periods where prednisone dose exceeds 20 mg/day for longer than 1 month, especially if the patient has associated T cell defects or is receiving other cytotoxic drugs or anti-TNF agents. Some experts also recommend monitoring CD4 cell counts in immunocompromised, non-HIV patients, using the threshold of 200 CD4 cells/mm3 for determining the need for PJP prophylaxis [13]. Most patients diagnosed with PJP have a defect in T cell immunity, whether from a disease (HIV infection, hematologic malignancy) or from immunosuppressive therapy, especially with corticosteroids [5, 11, 16]. In this regard, our study is similar to a previous study of PJP in non-HIV-infected patients, where: (1) underlying hematologic malignancies account for the majority of predisposing diseases; (2) corticosteroid use is a very common risk factor, found in 90 % of cases in our study (63/70), as also in cohorts not restricted to ICU patients (e.g., 204 of the 264 non-HIV-infected patients diagnosed with PJP at the Memorial Sloan-Kettering Cancer Center during the years 1963–1992 [16]).
The analysis of the risk factors for mortality in cohorts of non-HIV-infected patients with PJP has mostly identified the usual markers associated with death in ICU patients with respiratory diseases: mechanical ventilation [18], severity scores [20], ARDS, and shock (current study). However, the identification of these ‘obvious’ prognosis markers is of limited consequences, as these are mostly non-modifiable factors already present by the time patients are admitted to the ICU. Of more interest is the identification of prognosis markers that are modifiable. Of these, Festic et al. identified ‘intubation delay’ [20], with a mean time from ICU admission to intubation of 4 h in patients who survived, as compared to 48 h in patients who died (p = 0.03). This finding lead the authors to recommend early intubation and institution of mechanical ventilation on admission to the ICU for patients with ARF due to non-HIV-related PJP [20]. In the study presented herein, the identification of HSV or CMV on BAL was associated with in-ICU mortality on univariate analysis (p = 0.0031). This association may be causal, as herpes viruses are able to induce specific lesions of the lower respiratory tract, hence, worsening the lung damages caused by PJP [21, 22]. Unfortunately, due to the high risk of severe complications associated with lung biopsies in these patients (e.g., pneumothorax [20]), no samples were available for histological studies. In addition, herpes viruses-related cytopathogenic effect was not investigated on BAL in our center during the study period. Hence, we cannot exclude that the association between the presence of herpes viruses on BAL and mortality may be due to confusion bias. Indeed, it is conceivable that herpes viruses replication in the respiratory tract would merely be a marker of severe immunosuppression and/or severe ARDS, without playing a significant role in the pathways of PJP and in the outcome. Sepkowitz stated, in a landmark review on PJP in non-HIV-infected patients published almost two decades ago, that “in patients who are responding slowly or not at all, early repeat bronchoscopy should be considered to exclude the presence of additional pathogens, particularly CMV” [16]. Although the association between CMV or HSV on BAL and mortality observed in our study is in accordance with Sepkowitz’s statement, it does not constitute evidence that CMV or HSV directly contribute to patients’ death, especially given that the presence of CMV or HSV in BAL was no longer predictive of death in the multivariate analysis. In addition, given the observational design of our study, its findings are limited by the heterogeneity of the methods used for the diagnosis of herpes virus infection over the study period (1990–2010). This represents a potential classification bias.
In a monocentric cohort of non-HIV-infected patients with PJP, non-restricted to ICU patients (n = 78, including 58 documented), Arend et al. found that concomitant pulmonary infection, mostly bacterial pneumonia and CMV, was present in 35 % of the patients, and was associated with an increased risk of death, with a relative risk of 4.5 (95 % CI, 1.5–11.0, p = 0.01) [18]. In Festic et al.’s study, the presence of microorganisms on Gram stains or cultures was not associated with mortality (p = 0.16), but testing for viruses on respiratory specimens was not mentioned in this particular study [20]. Lastly, Kim et al. found no association between the identification of CMV on BAL and prognosis (morbidity and mortality) in a cohort of 106 non-HIV-infected patients with PJP not restricted to ICU patients, and concluded that it is not essential to administer an anti-CMV regimen when CMV is co-isolated from the BAL in patients with PJP [17]. However, only a randomized controlled trial would adequately address this question, but this is unlikely to occur, because of the low incidence of PJP in non-HIV-infected patients. Meanwhile, given the bad prognosis associated with herpes virus coinfection in non-HIV-infected patients with PJP and the availability of antiviral agents active against CMV or HSV, many experts would recommend treating these viruses if identified on BAL in immunocompromised patients with PJP.
The benefits of corticosteroids as adjunctive treatment for PJP have been clearly established in HIV-infected patients [12–14] through three randomized clinical trials of different designs, all reaching similar conclusions (e.g., a 50 % reduction in mortality rates in the largest study) [2]. In non-HIV-infected patients with severe PJP, the jury is still out: Pareja et al. found that doses >60 mg of equivalent prednisone per day were associated with a lower duration of mechanical ventilation and length of stay in the ICU [12]. However, there was no impact on mortality and in the need for ETI. More recently, Delclaux et al. found no significant benefit associated with adjunctive corticosteroids in terms of the need for mechanical ventilation and mortality [14]. Both studies were observational and of limited sample size (n = 31 and n = 30, respectively). Hence, there is currently neither evidence in favor of nor against adjuvant corticosteroid therapy in this setting. In our study, mortality was similar in patients with or without adjuvant corticosteroids, but we were limited by sample size and observational design. Again, only randomized controlled trials would appropriately address this issue, but this would require a large, multicentric, adequately powered clinical trial. The low incidence of PJP in non-HIV-treated patients and the heterogeneity of patients affected are some of the obstacles that these trials would have to face. Taking into account the low probability that such a trial will be undertaken in the near future, the ATS recently stated that “In patients without HIV with moderate to severe PJP, we suggest adding corticosteroids to the therapeutic regimens, using dosing regimens as advised for patients with AIDS,” a recommendation graded B-II, i.e., with moderate evidence to support a recommendation for use [13].
In conclusion, we found that the prognosis of HIV-negative immunocompromised patients with PJP admitted to the ICU because of ARF remains dismal, with an in-ICU mortality of 52.9 % overall, rising to 86.8 % in patients with ARDS. Co-infection with HSV or CMV was associated with an increased risk of death, although all co-infected patients in this study were treated with antiviral agents.
References
Wachter RM, Luce JM, Turner J, Volberding P, Hopewell PC (1986) Intensive care of patients with the acquired immunodeficiency syndrome. Outcome and changing patterns of utilization. Am Rev Respir Dis 134(5):891–896
Bozzette SA, Sattler FR, Chiu J, Wu AW, Gluckstein D, Kemper C, Bartok A, Niosi J, Abramson I, Coffman J, Hughlett C, Loya R, Cassens B, Akil B, Meng T-C, Boylen T, Nielsen D, Richman DD, Tilles JG, Leedom J, McCutchan A (1990) A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med 323(21):1451–1457
Gagnon S, Boota AM, Fischl MA, Baier H, Kirksey OW, La Voie L (1990) Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med 323(21):1444–1450
Briel M, Bucher HC, Boscacci R, Furrer H (2006) Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV-infection. Cochrane Database Syst Rev 3:CD006150
Mansharamani NG, Garland R, Delaney D, Koziel H (2000) Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 118(3):704–711
Morris A, Creasman J, Turner J, Luce JM, Wachter RM, Huang L (2002) Intensive care of human immunodeficiency virus-infected patients during the era of highly active antiretroviral therapy. Am J Respir Crit Care Med 166(3):262–267
Benito N, Moreno A, Miro JM, Torres A (2012) Pulmonary infections in HIV-infected patients: an update in the 21st century. Eur Respir J 39(3):730–745
Sepkowitz KA (2002) Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis 34(8):1098–1107
Sepkowitz KA, Brown AE, Armstrong D (1995) Pneumocystis carinii pneumonia without acquired immunodeficiency syndrome. More patients, same risk. Arch Intern Med 155(11):1125–1128
Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D (1992) Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 267(6):832–837
Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, Miceli C, Bourée P, Richard C (2008) Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care 12(1):R28
Pareja JG, Garland R, Koziel H (1998) Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest 113(5):1215–1224
Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA; American Thoracic Society Fungal Working Group (2011) An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 183(1):96–128
Delclaux C, Zahar JR, Amraoui G, Leleu G, Lebargy F, Brochard L, Schlemmer B, Brun-Buisson C (1999) Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin Infect Dis 29(3):670–672
Olsson M, Strålin K, Holmberg H (2001) Clinical significance of nested polymerase chain reaction and immunofluorescence for detection of Pneumocystis carinii pneumonia. Clin Microbiol Infect 7(9):492–497
Sepkowitz KA (1993) Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis 17(Suppl 2):S416–S422
Kim T, Moon SM, Sung H, Kim MN, Kim SH, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO (2012) Outcomes of non-HIV-infected patients with Pneumocystis pneumonia and concomitant pulmonary cytomegalovirus infection. Scand J Infect Dis (in press)
Arend SM, Kroon FP, van’t Wout JW (1995) Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch Intern Med 155(22):2436–2441
Green H, Paul M, Vidal L, Leibovici L (2007) Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 3:CD005590
Festic E, Gajic O, Limper AH, Aksamit TR (2005) Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest 128(2):573–579
Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J (2007) Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med 175(9):935–942
Bruynseels P, Jorens PG, Demey HE, Goossens H, Pattyn SR, Elseviers MM, Weyler J, Bossaert LL, Mentens Y, Ieven M (2003) Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet 362(9395):1536–1541
Acknowledgments
The authors thank the patients who participated in the study, the health care workers who took care of them in the ICU, and all the staff from the microbiology department.
Conflict of interest
The authors have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Le Tulzo and P. Tattevin co-directed this work.
Rights and permissions
About this article
Cite this article
Fillatre, P., Chevrier, S., Revest, M. et al. Human herpes virus co-infection is associated with mortality in HIV-negative patients with Pneumocystis jirovecii pneumonia. Eur J Clin Microbiol Infect Dis 32, 189–194 (2013). https://doi.org/10.1007/s10096-012-1730-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1730-7