Abstract
Background
Pneumocystis jirovecii pneumonia (PJP) remains a severe disease associated with high rates of invasive mechanical ventilation (MV) and mortality. The objectives of this study were to assess early risk factors for severe PJP and 90-day mortality, including the broncho-alveolar lavage fluid cytology profiles at diagnosis.
Methods
We prospectively enrolled all patients meeting pre-defined diagnostic criteria for PJP admitted at Nantes university hospital, France, from January 2012 to January 2017. Diagnostic criteria for PJP were typical clinical features with microbiological confirmation of P. jirovecii cysts by direct examination or a positive specific quantitative real-time polymerase chain reaction (PCR) assay. Severe PJP was defined as hypoxemic acute respiratory failure requiring high-flow nasal oxygen with at least 50% FiO2, non-invasive ventilation, or MV.
Results
Of 2446 respiratory samples investigated during the study period, 514 from 430 patients were positive for P. jirovecii. Of these 430 patients, 107 met criteria for PJP and were included in the study, 53 (49.5%) patients had severe PJP, including 30 who required MV. All patients were immunocompromised with haematological malignancy ranking first (n = 37, 35%), followed by solid organ transplantation (n = 27, 25%), HIV-infection (n = 21, 20%), systemic diseases (n = 13, 12%), solid tumors (n = 12, 11%) and primary immunodeficiency (n = 6, 8%). By multivariate analysis, factors independently associated with severity were older age (OR, 3.36; 95% CI 1.4–8.5; p < 0.05), a P. jirovecii microscopy-positive result from bronchoalveolar lavage (BAL) (OR, 1.3; 95% CI 1.54–9.3; p < 0.05); and absence of a BAL fluid alveolitis profile (OR, 3.2; 95% CI 1.27–8.8; p < 0.04). The 90-day mortality rate was 27%, increasing to 50% in the severe PJP group. Factors independently associated with 90-day mortality were worse SOFA score on day 1 (OR, 1.05; 95% CI 1.02–1.09; p < 0.001) whereas alveolitis at BAL was protective (OR, 0.79; 95% CI 0.65–0.96; p < 0.05). In the subgroup of HIV-negative patients, similar findings were obtained, then viral co-infection were independently associated with higher 90-day mortality (OR, 1.25; 95% CI 1.02–1.55; p < 0.05).
Conclusions
Older age and P. jirovecii oocysts at microscopic examination of BAL were independently associated with severe PJP. Both initial PJP severity as evaluated by the SOFA score and viral co-infection predicted 90-day mortality. Alveolitis at BAL examination was associated with less severe PJP. The pathophysiological mechanism underlying this observation deserves further investigation.
Similar content being viewed by others
Background
Over the last 10 years, survival benefits provided by steady advances in antitumor chemotherapy and immunosuppressant regimens for patients with autoimmune diseases, haematological malignancies, and solid organ transplants have substantially increased the number of adults living with immunodeficiencies [1, 2]. Among opportunistic infections in immunocompromised adults, Pneumocystis jirovecii pneumonia (PJP) was associated with high rates of intubation and mortality [3]. Consequently, an early identification and optimal treatment of patients with PJP remains a key priority [4, 5].
Since the advent of antiretroviral therapy, the incidence and mortality rates of PJP among patients positive for the human immunodeficiency virus (HIV) have decreased steadily [6]. However, PJP is being increasingly diagnosed in HIV-negative patients, in whom it carries a poorer prognosis [7, 8]. A higher proportion of neutrophils in broncho-alveolar lavage (BAL) fluid during PJP was associated with higher risks of respiratory complications and mortality [9, 10]. In the same way, a low lymphocyte count in BAL fluid was a risk factor for the failure of trimethoprim/sulfamethoxazole (TMP/SMZ) therapy [11]. Early adjunctive steroid therapy for severe PJP dramatically decreased mortality rates in HIV-positive patients [12, 13] but had variable effects in their HIV-negative counterparts [14,15,16]. These findings suggest that the immunological status and underlying diagnosis may influence the pathophysiology of PJP and the risk of mortality [17, 18]. However, BAL fluid cytology profiles have not been adequately evaluated as potential prognostic factor and predictors of treatment responses.
The identification of early predictors of PJP outcomes, including BAL fluid findings, may help to determine which patients are most likely to benefit from intensive care and could justify adjunctive steroid therapy. The aim of this prospective study of patients with PJP was to identify early risk factors for severe PJP and 90-day mortality.
Methods
Study design and participants
This is a retrospective analysis of prospective cohort. From January 2012 to January 2017, all patients presenting an invasive fungal infection that has been diagnosed in our center have been included in a prospective registry (the French prospective surveillance programme, RESSIF network), thus PJP patients have been included in a sub-cohort. For each patient with a positive sample, the following clinical findings were prospectively investigated: dyspnea and/or cough in immunocompromised patients with interstitial syndrome by radiography or CT scan. Among all respiratory samples investigated for 6 years (n = 2446), all positive tests for Pneumocystis jirovecii samples (n = 430) were assessed by a biologist and a clinician to investigate criteria for PJP and include them in this prospective cohort. The following data were prospectively collected: age; sex; underlying disease; PJP prophylaxis; other medications including glucocorticoids taken during the past month; type of symptoms and symptom duration at PJP diagnosis and time from symptom onset to hospital admission. Secondary, the clinical data were collected for all patients from medical records: laboratory findings (white blood cell count; absolute neutrophil count; C-reactive protein [CRP] level; BAL fluid findings including the cell profile assessed by an independent cytologist on centrifuged BAL fluid samples prepared with the Wright-Giemsa and Perls stains to allow the determination of macrophage, lymphocyte, neutrophil, eosinophil, and basophil counts); presence of P. jirovecii and/or other fungi and/or bacteria and/or viruses (influenza viruses, respiratory syncytial virus, adenovirus, cytomegalovirus) were recorded at PJP diagnosis. The SOFA score on day 1 and the ratio of the arterial partial pressure of oxygen over the fraction of inspired oxygen (PaO2/FiO2) on day 1 [19] were recorded for each patient at PJP diagnosis. Anti-PJP medications, adjuvant glucocorticoid therapy, oxygen supplementation, and ventilatory support provided at admission were documented. Finally, patient outcomes including 90-day mortality were recorded. The collection of follow-up data ended in December 2017.
Definitions
Severe PJP was defined as hypoxemic acute respiratory failure requiring high-flow nasal oxygen with at least 50% FiO2, non-invasive ventilation, or MV. Because not all patients were hospitalized in intensive care units, we chose this pragmatic and reproducible severity criterion. According to the Berlin definition [20], severe acute respiratory distress syndrome (ARDS) was defined as the presence of the following criteria within 3 days after ICU admission: new respiratory symptoms, bilateral opacities on chest radiographs or by CT, absence of suspected hydrostatic/cardiogenic pulmonary oedema, and PaO2/FiO2 ≤ 300. Lymphocytic alveolitis [21] was defined as a BAL fluid cell population containing more than 10% of lymphocytes and more than 5% of neutrophils combined with an activated macrophage phenotype, based on the diagnostic criteria for hypersensitivity pneumonitis, a condition characterised by alveolitis and migration to the alveoli of multiple cell types including activated T cells, monocytes, and natural killer cells [22]. Finally, patients with other pathogens associated with P. jirovecii in respiratory or blood samples were classified as having co-infection at ICU admission.
Statistical analysis
Patient characteristics were described using mean ± SD for continuous variables (or 95% confidence interval [95% CI] when appropriate) and proportions for qualitative variables. Continuous variables were compared using the Wilcoxon rank-sum and Kruskal–Wallis tests and qualitative variables using Fisher’s exact test with computation of the odds ratios (ORs) and their 95% CIs. Overall survival was assessed using Kaplan–Meier curves and log-rank tests. Factors associated with severity were identified by logistic regression analysis. Factors associated with all-cause 90-day mortality were identified by logistic regression analysis of patients alive after day 90 versus patients who died before day 90. Only factors reaching statistical significance (p < 0.05), with no more than 10% missing data, were included in the multivariate model. When collinearity between co-variates was detected, only the variable with the highest OR was kept into the multivariate regression model. Results are reported as log-transformed coefficients with their 95% CIs. Missing data were not interpolated. Analyses were performed on the whole population, then excluding HIV+ patients.
Results
Patient characteristics
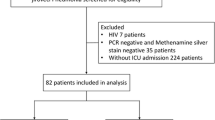
Of the 2446 respiratory samples tested during the study period, 514 from 430 patients were positive for P. jirovecii. Of these 430 patients, 107 met our PJP criteria and were included in the study; Table 1 reports their main characteristics. The remaining 323 patients were classified as having bronchial P. jirovecii colonisation (Fig. 1). The mean number of patients included per year was 21 and the number of patients per year increased gradually over time to reach a peak of 28 patients in 2015 (Additional file 1: Figure S1).
Of the 107 patients with PJP, 97 had positive BAL fluid, by a direct examination of BAL fluid in 49 patients and only detected by PCR in 48 patients. The induced sputum test was positive in 16 patients (by PCR, n = 15; and/or Grocott-Gomori stain, n = 3). Both the BAL fluid and the induced sputum test were positive in 6 patients. All patients were immunocompromised with haematological malignancy ranking first (n = 37, 35%), followed by solid organ transplantation (n = 27, 25%), HIV-infection (n = 21, 20%), systemic diseases (n = 13, 12%), solid tumors (n = 12, 11%) and primary immunodeficiency (n = 6, 8%). The mean HIV viral load at PJP diagnosis was 317,240 copies for HIV-positive patient. Only 21 (19.6%) patients were given PJP prophylaxis. Of these, 7 were compliant with the prescription during the last 2 months before diagnosis, which never consisted in TMP/SMZ.
The first-line PJP therapy was TMP/SMZ for 100 (93.5%) patients, 6 (5.6%) patients being treated with atovaquone; no PJP therapy was given to the remaining patient, who died within 24 h after ICU admission (PJP diagnosis was made post-mortem). Adjunctive glucocorticoid therapy was given to 61 (57%) patients based on severity criteria. Sixteen patients were switched from TMP-SMZ to atovaquone (n = 14) or pentamidine (n = 2), due to acute kidney injury (n = 9), myelotoxicity (n = 5) allergy (n = 1), or hepatic cytolysis (n = 1); 2 of these 16 patients had both acute kidney injury and myelotoxicity.
Of 97 patients with available BAL fluid cytology results, 31 (32%) had evident alveolitis profile. Mean BAL fluid percentages in the 97 patients were 21.7 ± 21.3 for neutrophils, 46 ± 25.7 for macrophages, and 32 ± 24.7 for lymphocytes. Bacterial co-infection was diagnosed in 19 (18%) patients, viral co-infection in 37 (35%) patients, and fungal co-infection in 5 (5%) patients (Additional file 1: Table S1). Lymphocytes and neutrophils mean ratio were, respectively, 22% and 23% in HIV patients, 34% and 21% in non-HIV patients, 22% and 30% in dead patients, 35% and 19% in survivors patients, 36% and 21% with only PJP patients, 29% and 22% during coinfection. We did not observe in our study eosinophilic and neutrophilic alveolitis.
Factors associated with severity and 90-day mortality
Of the 107 patients, 53 (49.5%) were classified as severe PJP (Table 2). ICU admission was required in 50 patients, including 30 who received MV. The HIV serology was positive in 6/53 (11%) patients with severe PJP and 15/54 (28%) patients with non-severe PJP.
Early risk factors associated with severity in the univariate analyses were age > 55 years (OR, 2.6; 95% CI 1.12–6.3; p < 0.02), albuminemia < 27 g/L (OR, 3.3; 95% CI 1.25–9; p < 0.001), blood neutrophil count > 6.5 G/L (OR, 6.5; 95% CI 2.4–20; p < 0.001), BAL fluid neutrophils > 12% (OR, 5.7; 95% CI 2–18; p < 0.001), P. jirovecii oocysts observed at direct examination of BAL fluid (OR, 2.8; 95% CI 1.2–6.9; p < 0.01), higher baseline lactic dehydrogenase value (OR, 1.35; 95% CI 1.13–1.68; p < 0.002), and higher CRP (OR, 1.01; 95% CI 1.01–1.02; p < 0.001). A BAL alveolitis profile was protective (OR, 0.3; 95% CI 0.1–0.8; p < 0.01). By multivariate analysis, factors independently associated with severe PJP were older age (OR, 3.36; 95% CI 1.4–8.5; p < 0.05), P. jirovecii oocysts observed at direct examination of BAL fluid (OR, 1.3; 95% CI 1.54–9.3; p < 0.05) and the absence of a BAL fluid alveolitis profile (OR, 3.2; 95% CI 1.27–8.8; p < 0.04).
Two factors were independently associated with 90-day mortality by multivariate analysis, a worse SOFA score was associated with higher 90-day mortality (OR, 1.05; 95% CI 1.02–1.09; p < 0.001), whereas BAL fluid alveolitis profile was associated with lower 90-day mortality (OR, 0.79; 95% CI 0.65–0.96; p < 0.05) (Table 3). HIV serology was a protective factor in univariate analysis but was not statistically associated with protective factor in the multivariate 90-day mortality analysis. In survival analysis HIV patients presenting with PJP was associated with statistically better prognostic than that of patients with hematologic diseases or solid cancer (Additional file 1: Figures S2, S3).
In the subgroup of HIV-negative patients, similar findings were obtained, then viral co-infection were independently associated with higher 90-day mortality (OR, 1.25; 95% CI 1.02–1.55; p < 0.05) (Additional file 1: Tables S2, S3). Factors associated with 90-day mortality in ICU patients were SOFA score and non-HIV patients (Additional file 1: Table S4).
Discussion
In this prospective study, over four-fifths of PJP patients were HIV-negative, and half met our criteria for severe disease. A worse SOFA score on admission and viral co-infection were independently associated with higher 90-day mortality in both whole patients and HIV-negative patients. Importantly, a BAL fluid cytological profile consistent with alveolitis was associated with lower 90-day mortality.
Severe PJP on admission as defined for our study was associated with a 56% risk of receiving MV, in keeping with recent results from large cohort studies [3, 23]. Given the prognostic significance of severity, an improved knowledge of early risk factors for severity is helpful to identify patients requiring more intensive monitoring and treatment.
As illustrated here, HIV patients less often experienced severe PJP compared to their HIV-negative counterparts, in agreement with earlier data [5, 8, 24,25,26,27]. The reduced severity of PJP in HIV-positive patients is more likely to be associated with the particularity of HIV-induced immunosuppression, of which pneumocystis is a hallmark of a severe adaptive cellular impairment. The co-morbidities in non-HIV patients (onco-hematology, solid organ transplantation and system diseases) may also contribute to their greater vulnerability. The immune recovery allowed by the initiation of antiretroviral therapy is probably correlated with better long-term outcomes in HIV-positive patients than in non-HIV patients whose profound immunosuppression is more frequently extended. HIV-positive patients also have lower neutrophil counts in BAL fluid samples [28], are less likely to develop severe PJP, and have lower mortality rates compared to HIV-negative patients [11, 26,27,28,29]. The SOFA score, viral co-infection and absence of alveolitis remained independently associated with a 90-day higher mortality in HIV-negative patients, suggesting that it is a strong independent marker in the whole PJP population.
By univariate analysis, early risk factors for severity in HIV-negative patients were markers for vulnerability (older age and lower serum albumin) and for inflammation (systemic inflammatory syndrome characterized by blood and alveolar polynucleosis serum CRP level). The patient subgroup at the most severe end of the spectrum had the highest CRP levels and neutrophil counts, suggesting that anti-inflammatory treatments might improve patient outcomes. Our study suggests that future prospective studies on adjunctive treatments for PJP should focus on HIV-negative patients, notably solid organ and haematological stem cell transplant recipients, meeting criteria for severe PJP (e.g., SOFA score > 2 with a low PaO2/FiO2 ratio). The potential relevance of a BAL fluid cytology profile consistent with alveolitis should probably also be taken into account when assessing the efficacy of new treatment regimens.
As expected, mortality within the first 90 days was significantly higher in patients with severe versus non-severe PJP. The independent association between a worse SOFA score at admission and 90-day mortality confirms the appropriateness of an evaluation with an intensivist to consider ICU admission of patients with oxygen-dependent PJP [30]. The quickSOFA, which is a simplified score based on three criteria, is easy to determine by non-intensivists and may be useful for determining when advice from an intensivist should be sought [31]. In addition to a worse SOFA score, viral co-infection at the time of PJP diagnosis was associated with higher 90-day mortality. Viral infections consisted mostly (67%) in reactivation of latent viruses (cytomegalovirus, herpes simplex virus, or Epstein-Barr virus), suggesting that this subgroup may have been characterised by a more profound immune deficiency. Taken together, this finding supports the hypothesis that the outcome of PJP is also closely related to the underlying diagnosis and immune response.
An important finding from this study is the clear association between a BAL fluid cytology profile consistent with alveolitis (> 10% lymphocytes, > 5% neutrophils, and presence of activated macrophages) and less severe PJP and lower 90-day mortality. The presence of neutrophils in BAL fluid has often been noted in clinical and experimental studies of acute respiratory distress syndrome (ARDS) [32, 33]. Three patient subgroups can be identified in our population, one defined by alveolitis, the other one PJP occuring in HIV-positive individuals, both having a better prognosis and the last one defined by a SOFA score above 2 on admission who have a more severe prognosis. Taken together, these results suggest that specific immunological characteristics might allow the identification of patient subgroups with different treatment needs and outcomes.
CD4+ T cell levels were non-significantly higher in patients with alveolitis, whereas glucocorticoid exposure was comparable in the two groups. To our knowledge, alveolitis during PJP has not been described as a good prognostic factor yet [21]. In murine models of CD4+ T-cell depleted mice, increased alveolar recruitment of CD8+ T cells induced by interleukin-7 therapy improved P. jirovecii clearance [34]. Lymphocyte count in BAL fluid is increased in patients with alveolitis, which was associated with a better prognosis in our study. Thus, alveolitis during PJP might reflect maintenance of an effective pulmonary immune response including CD8+ T-cell recruitment. Glucocorticoid therapy might, therefore, be unnecessary in patients with PJP and alveolitis, although their specific response to glucocorticoids has not been investigated to date.
Four-fifths of our patients had not been prescribed PJP prophylaxis, and among those with a prescription only one-third were compliant. These findings confirm earlier results [16, 35] and further identify the absence of TMP/SMZ prophylaxis as a major risk factor for PJP in high-risk patients [29]. Providing appropriate prophylactic anti-microbial treatments to patients with immunosuppression, notably related to haematological diseases and transplantation, is crucial to improve patient outcomes. Adherence to prophylactic treatment must be supported at each follow-up visit. PJP usually develops in patients with CD4+counts below 300/mm3 [36,37,38], although the depth of lymphopenia does not correlate with PJP severity [39, 40]. A history of glucocorticoid exposure is an often reported risk factor for PJP in HIV-negative patients [41, 42]. Among our patients, over half were receiving glucocorticoid therapy at the diagnosis of PJP, in a mean prednisone-equivalent dosage of 20 mg/day. Adjuvant glucocorticoid therapy was given to most severe patients who failed antibiotic treatment alone. Given the history of glucocorticoid exposure, the effects of adjuvant glucocorticoid therapy were difficult to assess. The role for adjuvant glucocorticoid therapy in patients with PJP is debated [26, 43,44,45,46,47] and is currently being assessed in a prospective study (ClinicalTrials.gov Identifier: NCT02944045).
Serum LDH level at PJP diagnosis was associated with poor outcomes in HIV-positive and HIV-negative patients in several studies [48]. In our population, LDH > 7 µkat/L (420 U/L) levels were associated with severe PJP by univariate analysis but not with higher 90-day mortality by multivariate analysis. Missing data or a role for unidentified confounders may explain this result.
A limitation of our study is the use of non-validated criteria for defining severe PJP. The European Conference on Infections in Leukaemia-5 group has defined severe PJP, like those defined for HIV-positive patients [49] such as PaO2 less than 8 kPa, oxygen saturation less than 91%, and radiological impairment. These criteria were only used in retrospective study of PJP patients [45]. However, these criteria have not been prospectively validated in non-HIV patients. HIV-patients less often experienced severe PJP compared to their HIV-negative counterparts, enhancing the need to propose an early severity scale adapted to this specific population. We, therefore, relied on criteria indicating hypoxemic ARF, namely, FiO2 ≥ 50% or NIV or MV. The strong association of severe PJP with higher 90-day mortality indicates that our definition successfully identified severe PJP.
Conclusion
In a large prospective cohort of patients admitted for PJP, the SOFA score on admission and presence of viral co-infection were independently associated with higher 90-day mortality. Importantly, a BAL fluid cytology profile suggesting alveolitis was associated with less severe PJP and lower 90-day mortality. Our findings, notably the associations linking the SOFA score and alveolitis to 90-day mortality, deserve further evaluation in a multicentre prospective study. Future studies of adjunctive treatments for PJP therapy should differentiate between patients with and without alveolitis.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
severe acute respiratory distress syndrome
- BAL:
-
broncho-alveolar lavage
- BMI:
-
body mass index
- CI:
-
confidence interval
- CMV:
-
cytomegalovirus
- CRP:
-
C-reactive protein
- EBV:
-
Epstein–Barr virus
- FiO2 :
-
fraction of inspired oxygen
- HIV:
-
human immunodeficiency virus
- HSV1:
-
herpes simplex virus
- ICU:
-
intensive care unit
- LDH:
-
lactate dehydrogenase
- MV:
-
invasive mechanical ventilation
- NIV:
-
non-invasive ventilation
- OR:
-
odds ratio
- PaO2 :
-
arterial partial pressure of oxygen
- PCR:
-
real-time polymerase chain reaction
- PJP:
-
Pneumocystis jirovecii pneumonia
- RSV:
-
respiratory syncytial virus
- SAPS2:
-
simplified acute physiology score version 2
- SD:
-
standard deviation
- SOFA score:
-
sequential organ failure assessment score
- TMP-SMX:
-
trimethoprim–sulfamethoxazole
References
Fernández-Ruiz M, Kumar D, Humar A. Clinical immune-monitoring strategies for predicting infection risk in solid organ transplantation. Clin Transl Immunol. 2014;3(2):e12.
Winthrop KL, Novosad SA, Baddley JW, Calabrese L, Chiller T, Polgreen P, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis. 2015;74(12):2107–16.
Azoulay E, Pickkers P, Soares M, Perner A, Rello J, Bauer P, et al. Acute hypoxemic respiratory failure in immunocompromised patients: the Efraim multinational prospective cohort study. Intensive Care Med. 2017;43:1808–19.
Novosad SA, Winthrop KL. Beyond tumor necrosis factor inhibition: the expanding pipeline of biologic therapies for inflammatory diseases and their associated infectious sequelae. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58(11):1587–98.
Ko Y, Jeong B-H, Park HY, Koh W-J, Suh GY, Chung MP, et al. Outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. J Crit Care. 2014;29(3):356–61.
Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34(8):1098–107.
Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128(2):573–9.
Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, et al. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care Lond Engl. 2008;12(1):R28.
Jensen BN, Lisse IM, Gerstoft J, Borgeskov S, Skinhøj P. Cellular profiles in bronchoalveolar lavage fluid of HIV-infected patients with pulmonary symptoms: relation to diagnosis and prognosis. AIDS Lond Engl. 1991;5(5):527–33.
Lee JY, Park HJ, Kim YK, Yu S, Chong YP, Kim S-H, et al. Cellular profiles of bronchoalveolar lavage fluid and their prognostic significance for non-HIV-infected patients with Pneumocystis jirovecii pneumonia. J Clin Microbiol. 2015;53(4):1310–6.
Kim T, Sung H, Chong YP, Kim SH, Choo EJ, Choi SH, et al. Low lymphocyte proportion in bronchoalveolar lavage fluid as a risk factor associated with the change from trimethoprim/sulfamethoxazole used as first-line treatment for Pneumocystis jirovecii pneumonia. Infect Chemother. 2018;50(2):110–9.
Gagnon S, Boota AM, Fischl MA, Baier H, Kirksey OW, La Voie L. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med. 1990;323(21):1444–50.
Bozzette SA, Sattler FR, Chiu J, Wu AW, Gluckstein D, Kemper C, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1990;323(21):1451–7.
Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest. 1998;113(5):1215–24.
Delclaux C, Zahar JR, Amraoui G, Leleu G, Lebargy F, Brochard L, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients: retrospective study of 31 patients. Clin Infect Dis Off Publ Infect Dis Soc Am. 1999;29(3):670–2.
Zahar JR, Robin M, Azoulay E, Fieux F, Nitenberg G, Schlemmer B. Pneumocystis carinii pneumonia in critically ill patients with malignancy: a descriptive study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;35(8):929–34.
Limper AH, Offord KP, Smith TF, Martin WJ. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140(5):1204–9.
Azoulay E, Parrot A, Flahault A, Cesari D, Lecomte I, Roux P, et al. AIDS-related Pneumocystis carinii pneumonia in the era of adjunctive steroids: implication of BAL neutrophilia. Am J Respir Crit Care Med. 1999;160(2):493–9.
Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604–9.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Iriart X, Witkowski B, Courtais C, Abbes S, Tkaczuk J, Courtade M, et al. Cellular and cytokine changes in the alveolar environment among immunocompromised patients during Pneumocystis jirovecii infection. Med Mycol. 2010;48(8):1075–87.
Limongi F, Fallahi P. Hypersensitivity pneumonitis and alpha-chemokines. Clin Ter. 2017;168(2):e140–5.
Kojicic M, Li G, Hanson AC, Lee K-M, Thakur L, Vedre J, et al. Risk factors for the development of acute lung injury in patients with infectious pneumonia. Crit Care Lond Engl. 2012;16(2):R46.
Boonsarngsuk V, Sirilak S, Kiatboonsri S. Acute respiratory failure due to Pneumocystis pneumonia: outcome and prognostic factors. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2009;13(1):59–66.
Kim SJ, Lee J, Cho Y-J, Park YS, Lee C-H, Yoon HI, et al. Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J Infect. 2014;69(1):88–95.
Lemiale V, Debrumetz A, Delannoy A, Alberti C, Azoulay E. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res. 2013;14(1):87.
Roblot F, Le Moal G, Godet C, Hutin P, Texereau M, Boyer E, et al. Pneumocystis carinii pneumonia in patients with hematologic malignancies: a descriptive study. J Infect. 2003;47(1):19–27.
Iriart X, Witkowski B, Cassaing S, Abbes S, Menard S, Fillaux J, et al. Alveolar and blood T lymphocyte profiles in Pneumocystis jirovecii-positive patients: effects of HIV status. J Infect Dis. 2011;204(4):544–53.
Azoulay E, Roux A, Vincent F, Kouatchet A, Argaud L, Rabbat A, et al. A multivariable prediction model for Pneumocystis jirovecii pneumonia in hematology patients with acute respiratory failure. Am J Respir Crit Care Med. 2018.
Lamia B, Hellot M-F, Girault C, Tamion F, Dachraoui F, Lenain P, et al. Changes in severity and organ failure scores as prognostic factors in onco-hematological malignancy patients admitted to the ICU. Intensive Care Med. 2006;32(10):1560–8.
Tokioka F, Okamoto H, Yamazaki A, Itou A, Ishida T. The prognostic performance of qSOFA for community-acquired pneumonia. J Intensive Care. 2018;6:46.
Capelozzi VL, Allen TC, Beasley MB, Cagle PT, Guinee D, Hariri LP, et al. Molecular and immune biomarkers in acute respiratory distress syndrome: a perspective from members of the pulmonary pathology society. Arch Pathol Lab Med. 2017;141(12):1719–27.
Dhanireddy S, Altemeier WA, Matute-Bello G, O’Mahony DS, Glenny RW, Martin TR, et al. Mechanical ventilation induces inflammation, lung injury, and extra-pulmonary organ dysfunction in experimental pneumonia. Lab Investig J Tech Methods Pathol. 2006;86(8):790–9.
Ruan S, Samuelson DR, Assouline B, Morre M, Shellito JE. Treatment with interleukin-7 restores host defense against pneumocystis in CD4+ T-lymphocyte-depleted mice. Infect Immun. 2015;84(1):108–19.
Matsumura Y, Ito Y, Yamamoto M, Matsushima A, Nagao M, Takakura S, et al. Pneumocystis polymerase chain reaction and blood (1 → 3)-β-d-glucan assays to predict survival with suspected Pneumocystis jirovecii pneumonia. J Infect Chemother. 2014;20(2):109–14.
Roblot F, Le Moal G, Kauffmann-Lacroix C, Bastides F, Boutoille D, Verdon R, et al. Pneumocystis jirovecii pneumonia in HIV-negative patients: a prospective study with focus on immunosuppressive drugs and markers of immune impairment. Scand J Infect Dis. 2014;46(3):210–4.
Roblot F, Imbert S, Godet C, Kauffmann C, Ragot S, Le Moal G, et al. Risk factors analysis for Pneumocystis jirovecii pneumonia (PCP) in patients with haematological malignancies and pneumonia. Scand J Infect Dis. 2004;36(11–12):848–54.
Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg Infect Dis. 2014;20(9):1490–7.
Torres HA, Chemaly RF, Storey R, Aguilera EA, Nogueras GM, Safdar A, et al. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jirovecii pneumonia in cancer patients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2006;25(6):382–8.
Mansharamani NG, Balachandran D, Vernovsky I, Garland R, Koziel H. Peripheral blood CD4+ T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118(3):712–20.
Martin-Garrido I, Carmona EM, Specks U, Limper AH. Pneumocystis pneumonia in patients treated with rituximab. Chest. 2013;144(1):258–65.
Sepkowitz KA, Brown AE, Telzak EE, Gottlieb S, Armstrong D. Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA. 1992;267(6):832–7.
Ewald H, Raatz H, Boscacci R, Furrer H, Bucher HC, Briel M. Adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in patients with HIV infection. Cochrane Database Syst Rev. 2015;4:CD006150.
Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183(1):96–128.
Kofteridis DP, Valachis A, Velegraki M, Antoniou M, Christofaki M, Vrentzos GE, et al. Predisposing factors, clinical characteristics and outcome of Pneumonocystis jirovecii pneumonia in HIV-negative patients. J Infect Chemother Off J Jpn Soc Chemother. 2014;20(7):412–6.
Bollée G, Sarfati C, Thiéry G, Bergeron A, de Miranda S, Menotti J, et al. Clinical picture of Pneumocystis jirovecii pneumonia in cancer patients. Chest. 2007;132(4):1305–10.
Roblot F, Godet C, Le Moal G, Garo B, Faouzi Souala M, Dary M, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2002;21(7):523–31.
Schmidt JJ, Lueck C, Ziesing S, Stoll M, Haller H, Gottlieb J, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care Lond Engl. 2018;22(1):307.
Miller RF, Le Noury J, Corbett EL, Felton JM, De Cock KM. Pneumocystis carinii infection: current treatment and prevention. J Antimicrob Chemother. 1996;37(Suppl B):33–53.
Acknowledgements
We thank the clinicians and microbiologists who contributed to the management of the study patients for their commitment to providing optimal patient care.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
BJG and BT analysed and interpreted the data. CS performed the cytological examinations of broncho-alveolar fluid samples. FM, RAL, and BJG were members of the independent committee that adjudicated the PJP cases. BJG and JR wrote the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All databases were approved by the Institut Pasteur institutional review board (#2009-34/IRB) and by the French data protection authority (Commission pour l’Informatique et les Libertés, CNIL) and the collection of biological specimens was reported to the Ministry for Education and Research (#DC-2008-68 collection 12).
Consent for publication
All authors read the final version of the manuscript and approved its submission to Annals of Intensive Care.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional figures and tables.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gaborit, B.J., Tessoulin, B., Lavergne, RA. et al. Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann. Intensive Care 9, 131 (2019). https://doi.org/10.1186/s13613-019-0604-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-019-0604-x