Abstract
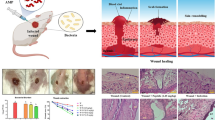
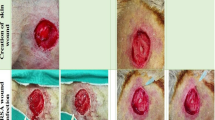
The aim of this study was to evaluate the efficacy of distinctin in the management of cutaneous methicillin-resistant Staphylococcus aureus (MRSA) wound infections in an experimental mouse model. Wounds, made in the panniculus carnosus of BALB/c mice, were inoculated with 5 × 107 colony-forming units (CFU) of MRSA. Mice were treated with topical distinctin (1 mg/kg of body weight), topical teicoplanin (7 mg/kg of body weight), intraperitoneal teicoplanin (7 mg/kg of body weight); topical teicoplanin and daily intraperitoneal teicoplanin; topical distinctin and daily intraperitoneal teicoplanin. Bacterial cultures of excised tissues and histological examination of microvessel density and of vascular endothelial growth factor (VEGF) expression were studied. It was found that topical distinctin combined with parenteral teicoplanin inhibited bacterial growth to levels comparable with those observed in uninfected animals. Wounded areas of animals treated with distinctin were characterized by a more mature granulation tissue, with a more organized and denser type of connective tissue, compared to mice treated only with teicoplanin. Treatment with topical distinctin had a significant impact on VEGF expression and microvessel density. The combined use of distinctin with teicoplanin may be useful in the management of infected wounds by significantly inhibiting bacterial growth and accelerating the repair process.


Similar content being viewed by others
References
Saleh K, Sonesson A, Persson B, Riesbeck K, Schmidtchen A (2011) A descriptive study of bacterial load of full-thickness surgical wounds in dermatologic surgery. Dermatol Surg 37(7):1014–1022
Lowy FD (2003) Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 111:1265–1273
Bone RC (1994) Gram-positive organisms and sepsis. Arch Intern Med 154:26–34
Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW (2005) A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother 55:143–149
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Pukala TL, Bowie JH, Maselli VM, Musgrave IF, Tyler MJ (2006) Host-defence peptides from the glandular secretions of amphibians: structure and activity. Nat Prod Rep 23(3):368–393
Rinaldi AC (2002) Antimicrobial peptides from amphibian skin: an expanding scenario. Curr Opin Chem Biol 6(6):799–804
Lipsky BA, Holroyd KJ, Zasloff M (2008) Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47:1537–1545
Batista CV, Scaloni A, Rigden DJ, Silva LR, Rodrigues Romero A, Dukor R, Sebben A, Talamo F, Bloch C (2001) A novel heterodimeric antimicrobial peptide from the tree-frog Phyllomedusa distincta. FEBS Lett 494(1–2):85–89
Cirioni O, Ghiselli R, Orlando F, Silvestri C, De Luca S, Salzano AM, Mocchegiani F, Saba V, Scalise G, Scaloni A, Giacometti A (2008) Efficacy of the amphibian peptide distinctin in a neutropenic mouse model of staphylococcal sepsis. Crit Care Med 36(9):2629–2633
Dalla Serra M, Cirioni O, Vitale RM, Renzone G, Coraiola M, Giacometti A, Potrich C, Baroni E, Guella G, Sanseverino M, De Luca S, Scalise G, Amodeo P, Scaloni A (2008) Structural features of distinctin affecting peptide biological and biochemical properties. Biochemistry 47(30):7888–7899
Lee IH, Lee YS, Kim CH, Kim CR, Hong T, Menzel L, Boo LM, Pohl J, Sherman MA, Waring A, Lehrer RI (2001) Dicynthaurin: an antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. Biochim Biophys Acta 1527:141–148
Jang WS, Kim KN, Lee YS, Nam MH, Lee IH (2002) Halocidin: a new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett 521:81–86
Yomogida S, Nagaoka I, Yamashita T (1996) Purification of the 11- and 5-kDa antibacterial polypeptides from guinea pig neutrophils. Arch Biochem Biophys 328:219–226
Scocchi M, Zelezetsky I, Benincasa M, Gennaro R, Mazzoli A, Tossi A (2005) Structural aspects and biological properties of the cathelicidin PMAP-36. FEBS J 272:4398–4406
Raimondo D, Andreotti G, Saint N, Amodeo P, Renzone G, Sanseverino M, Zocchi I, Molle G, Motta A, Scaloni A (2005) A folding-dependent mechanism of antimicrobial peptide resistance to degradation unveiled by solution structure of distinctin. Proc Natl Acad Sci USA 102(18):6309–6314
Jang WS, Kim CH, Kim KN, Park SY, Lee JH, Son SM, Lee IH (2003) Biological activities of synthetic analogs of halocidin, an antimicrobial peptide from the tunicate Halocynthia aurantium. Antimicrob Agents Chemother 47:2481–2486
Okuda D, Yomogida S, Tamura H, Nagaoka I (2006) Determination of the antibacterial and lipopolysaccharide-neutralizing regions of guinea pig neutrophil cathelicidin peptide CAP11. Antimicrob Agents Chemother 50:2602–2607
Jang WS, Lee SC, Lee YS, Shin YP, Shin KH, Sung BH, Kim BS, Lee SH, Lee IH (2007) Antimicrobial effect of halocidin-derived peptide in a mouse model of Listeria infection. Antimicrob Agents Chemother 51(11):4148–4156
Shin YP, Park HJ, Shin SH, Lee YS, Park S, Jo S, Lee YH, Lee IH (2010) Antimicrobial activity of a halocidin-derived peptide resistant to attacks by proteases. Antimicrob Agents Chemother 54(7):2855–2866
Lauer G, Sollberg S, Cole M, Flamme I, Stürzebecher J, Mann K, Krieg T, Eming SA (2000) Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 115:12–18
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9:777–794
Cianfarani F, Tommasi R, Failla CM, Viviano MT, Annessi G, Papi M, Zambruno G, Odorisio T (2006) Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol 154:34–41
Frantz S, Vincent KA, Feron O, Kelly RA (2005) Innate immunity and angiogenesis. Circ Res 96:15–26
Asai J, Takenaka H, Katoh N, Kishimoto S (2006) Dibutyryl cAMP influences endothelial progenitor cell recruitment during wound neovascularization. J Invest Dermatol 126:1159–1167
Shinzawa H, Takeda A, Sone Y, Murashita K, Uchinuma E (2007) Wound healing process of a full-thickness skin wound model in rats. Int Surg 92:63–72
Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H (2007) Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 127:594–604
Pace JL, Yang G (2006) Glycopeptides: update on an old successful antibiotic class. Biochem Pharmacol 71:968–980
Clinical and Laboratory Standards Institute (CLSI) (2003) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard M7-A6. CLSI, Villanova, PA
Eliopoulos GM, Moellering RC Jr (1996) Antimicrobial combinations. In: Lorian V (ed) Antibiotics in laboratory medicine, 4th edn. Williams & Wilkins, Baltimore, pp 330–393
Simonetti O, Cirioni O, Goteri G, Ghiselli R, Kamysz W, Kamysz E, Silvestri C, Orlando F, Barucca C, Scalise A, Saba V, Scalise G, Giacometti A, Offidani A (2008) Temporin A is effective in MRSA-infected wounds through bactericidal activity and acceleration of wound repair in a murine model. Peptides 29(4):520–528
Fung HB, Chang JY, Kuczynski SA (2003) Practical guide to the treatment of complicated skin and soft tissue infections. Drugs 63:1459–1480
Bergmann S, Hammerschmidt S (2007) Fibrinolysis and host response in bacterial infections. Thromb Haemost 98(3):512–520
[No authors listed] (1993) Proteolytic enzymes in coagulation, fibrinolysis, and complement activation. Part A. Mammalian blood coagulation factors and inhibitors. Methods Enzymol 222:1–559
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55
Vaara M, Porro M (1996) Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob Agents Chemother 40:1801–1805
Mangoni ML, Rinaldi AC, Di Giulio A, Mignogna G, Bozzi A, Barra D, Simmaco M (2000) Structure–function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur J Biochem 267:1447–1454
Rinaldi AC, Mangoni ML, Rufo A, Luzi C, Barra D, Zhao H, Kinnunen PK, Bozzi A, Di Giulio A, Simmaco M (2002) Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem J 368:91–100
Resende JM, Moraes CM, Munhoz VH, Aisenbrey C, Verly RM, Bertani P, Cesar A, Piló-Veloso D, Bechinger B (2009) Membrane structure and conformational changes of the antibiotic heterodimeric peptide distinctin by solid-state NMR spectroscopy. Proc Natl Acad Sci U S A 106(39):16639–16644
Martin P (1997) Wound healing—aiming for perfect skin regeneration. Science 276:75–81
Santoro MM, Gaudino G (2005) Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res 304:274–286
Werner S, Krieg T, Smola H (2007) Keratinocyte–fibroblast interactions in wound healing. J Invest Dermatol 127:998–1008
Naldini A, Carraro F (2005) Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy 4:3–8
Ho QT, Kuo CJ (2007) Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 39(7–8):1349–1357
Conflicts of interest and sources of funding
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 25 kb)
Rights and permissions
About this article
Cite this article
Simonetti, O., Cirioni, O., Ghiselli, R. et al. Antimicrobial properties of distinctin in an experimental model of MRSA-infected wounds. Eur J Clin Microbiol Infect Dis 31, 3047–3055 (2012). https://doi.org/10.1007/s10096-012-1663-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1663-1