Abstract
The high mutation rate of influenza virus, combined with the increasing worldwide use of influenza virus-specific drugs, allows the selection of viruses that are resistant to the currently available antiviral medications. Therefore, reliable tests for the rapid detection of drug-resistant influenza virus strains are required. We evaluated the use of a procedure involving real-time polymerase chain reaction (PCR) followed by melting point analysis (MPA) of hybrids formed between the PCR product and a specific oligonucleotide probe for the identification of point mutations in the influenza A virus neuraminidase gene (NA) that are associated with oseltamivir resistance [resulting in the amino acid change H275Y for seasonal and pandemic influenza A(H1N1) viruses and E119V for A(H3N2) viruses]. Therefore, 54 seasonal A(H1N1) (12 oseltamivir-resistant and 42 sensitive strains), 222 A(H1N1)2009 (5 resistant, 217 sensitive), and 51 A(H3N2) viruses (2 resistant, 49 sensitive) were tested by MPA, and the results were compared to those obtained by sequencing the NA gene. The results clearly indicate that the identification of drug resistance mutations by MPA is as accurate as sequencing, irrespective of whether MPA is performed using clinical material or the corresponding isolate. MPA enables a clear identification of mutations associated with antiviral resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza virus infections are associated with significant morbidity and mortality, and their prevention and treatment has become an increasing priority in many regions of the world [1–5]. The neuraminidase inhibitors (NIs) oseltamivir and zanamivir are efficient and potent antiviral drugs that are widely used for the treatment of influenza infections, but as with any antiviral compound, their therapeutic and prophylactic efficacy can be compromised by the emergence of drug-resistant mutants [6–10]. The worldwide emergence of resistant influenza A(H1N1) viruses during the influenza season 2007/2008 [11–13] underscores the importance of surveillance for resistant strains of influenza virus. Since NI resistance is usually caused by single-point mutations that lead to specific amino acid changes, sequencing the neuraminidase gene (NA) provides information about the NI susceptibility of circulating strains [14, 15]. Although functional assays and sequencing are well-established techniques that provide reliable results [16, 17], one of the drawbacks of their routine use for influenza surveillance is that NI resistance testing currently requires facilities that are only available in a limited number of well-equipped laboratories.
High-resolution melting curve analysis was introduced recently as a new detection method for single-nucleotide polymorphisms (SNPs), and it has been applied for the detection of cytomegalovirus UL97 mutations conferring ganciclovir resistance and for the detection of antigenic variants of influenza B viruses [18]. This technique is relatively easy to implement on widely used real-time polymerase chain reaction (PCR) platforms.
The aims of the present study were: (a) to develop a real-time PCR probe hybridization melting point analysis (MPA) protocol for the rapid identification of the most common point mutations in the neuraminidase gene of seasonal and pandemic influenza A(H1N1) viruses (H275Y mutation) and influenza A(H3N2) strains (E119V mutation) that are associated with oseltamivir resistance; (b) to evaluate this assay by testing 54 seasonal A(H1N1), 222 A(H1N1)2009, and 51 A(H3N2) influenza viruses with known NA gene sequences; and (c) to investigate whether MPA with influenza viruses derived directly from clinical material is feasible and provides reliable data.
Materials and methods
Clinical specimens and subtyping of influenza viruses
Seasonal A/Solomon Islands/3/2006-like (A(H1N1)) and A/Brisbane/10/2007-like (A(H3N2)) influenza A virus strains, as well as pandemic A(H1N1)2009 (A/California/7/2009-like) viruses, were obtained from nasopharyngeal swabs (NPS) collected from patients during the seasons 2007/08 to 2010/11 by sentinel physicians participating in the Austrian Influenza Network and sent to the Department of Virology, Medical University of Vienna, for detection, typing, and subtyping by PCR and virus isolation in cell culture as described previously [19–21]. Further antigenic characterization of isolates was performed by the hemagglutination inhibition test and genetic characterization was performed by sequencing the HA and the NA gene [19]. Influenza-virus-positive NPS and tissue culture supernatants were stored at −80°C until further analysis.
Antiviral resistance testing by sequencing the NA gene
For sequencing, the influenza viral RNA was isolated from tissue culture supernatant using a QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). Reverse transcription was performed using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. The following amplification by PCR was done using an Advantage HF 2 PCR Kit (BD Biosciences Clontech, Palo Alto, CA, USA). The primers used are listed in Table 1. Amplicons were purified using MultiScreen (Millipore, Bedford, MA, USA), sequenced using an ABI PRISM BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), and analyzed using an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Nucleotide sequences were aligned using ClustalW (EMBL-EBI, European Bioinformatics Institute).
Antiviral resistant reference strains used for MPA evaluation
Seasonal influenza A(H1N1) strains (A/Solomon Islands/3/2006-like) carrying the H275Y point mutation associated with oseltamivir resistance circulated in Austria during the 2007/08 season. Twelve strains were sent to the WHO Influenza Centre, MRC National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, UK (WHO CC London), where oseltamivir resistance was confirmed. These 12 strains were, therefore, considered as resistant A(H1N1) reference strains.
Resistant influenza strains of subtypes A(H1N1)2009 and A(H3N2) that were used for the evaluation of the assay were obtained from the following international collaborators: the resistant A(H3N2) reference strain A/Fukui/45/04 (E119V) was obtained from Dr. A. Hurt, Neuraminidase Inhibitor Susceptibility Network (NISN, http://www.nisn.org); the resistant A(H1N1)2009 virus A/Rhineland-Pfalz/86/2009 (H275Y) was obtained from Dr. B. Schweiger, Robert Koch Institute, National Reference Centre for Influenza in Germany; and the strains A/Texas/1/77 (A/H3N2 [E119V]) and A/Denmark/528/2009 (A(H1N1)2009 [H275Y]) were obtained from Vicki Gregory, WHO CC London.
Antiviral resistance testing by MPA
Three different real-time PCR assays were used for amplification and MPA of seasonal A(H1N1), A/H1N1 (2009), and A(H3N2) viruses. After amplification, the amplicons were allowed to form hybrids with a fluorescently labeled oligonucleotide probe covering the region, including the codon for H275 (A(H1N1) strains) or E119 (A(H3N2) strains). The melting point of the resulting hybrid was determined by slowly raising the temperature and observing changes in the fluorescence intensity. Hybrids containing a point mutation in the amplicon had a lower melting point due to the sequence mismatch with the probe.
Briefly, the MPA assay consists of five steps: (1) RNA extraction, (2) reverse transcription, (3) real-time PCR amplification of the region of interest, (4) hybridization of the amplicon with a specific oligonucleotide probe, and (5) determination of the melting point of the hybrid:
-
(1)
Extraction of influenza virus RNA from clinical material as well as from tissue culture supernatant was performed with Magna Pure LC2.0 using a MagNA Pure LC Total Nucleic Acid Isolation Kit according to the manufacturer’s instructions.
-
(2)
The reverse transcription reaction was performed using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA), as described above.
-
(3)
NA-specific real-time PCR was then performed in a LightCycler 480 (Roche Diagnostics, Mannheim, Germany) using the appropriate primers for specific amplification of the NA region of seasonal A(H1N1), A(H1N1)2009, or A(H3N2) viruses, as listed in Table 2. Briefly, 1 μl viral c-DNA was added to the following reaction mixture: 12.5 μl LightCycler 480 SYBR Green 1 Master (Roche Diagnostics, Mannheim, Germany), 0.2 μl forward primer (3.5 pmol), 1 μl reverse primer (17.5 pmol), 0.8 μl probe (8 pmol) (1.2 μl with 12 pmol for the A/H3N2-tests), and 9.5 μl water (9.1 μl for the A/H3N2-tests). The cycling conditions were: initial holds at 50°C for 3 min and 95°C for 10 min, followed by 45 cycles at 95°C for 15s, 60°C for 30s, and 77°C for 31s. The data were analyzed by using the Roche LightCycler software release 1.5.0 version 1.5.0.39. Volumes and cycler conditions were adapted to the protocols evaluated and used in our laboratory for the detection of respiratory viruses by real-time PCR.
Table 2 Primers and probes used for the detection of point mutations associated with oseltamivir resistance by melting point analysis (MPA) The detection limits of the NA-specific PCRs as determined by testing serial dilutions of samples with known viral load (WHO EQAP Panel 7 [22]) were as follows: 60 copies/ml for A(H1N1)-, 30 copies/ml for A(H1N1)2009-, and 20 copies/ml for A(H3N2)-NA-specific PCR.
-
(4 and 5)
MPA was then performed in the same instrument as follows: the samples were first held at 95°C for 1 s (ramp rate 4.4°C/s) and 35°C for 1 min (ramp rate 2.2°C/s), after which the temperature was continuously increased with a ramp rate of 0.06°C/s, with five acquisitions of fluorescence data per °C. Melting curve plots were calculated automatically using Roche LightCycler software, release 1.5.0, version 1.5.0.39. Fluorescence data were expressed as the negative of the first derivative of the fluorescence intensity over time (−d/dT) in order to display the midpoint of the melting curve as a peak.
Antiviral resistance testing by functional chemiluminescence assay
Functional testing for oseltamivir resistance was carried out for samples with mutations located within the primer region of the NA-specific PCRs not known to be associated with antiviral resistance. Testing was based on a chemiluminescence assay and was carried out using an NA-Star Influenza Neuraminidase Inhibitor Resistance Detection Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions.
Results
Detection of point mutations associated with antiviral resistance by sequence analysis of the NA gene of tissue-culture-grown viruses
The NA genes of 165 seasonal A(H1N1), 220 A(H1N1)2009, and 49 A(H3N2) influenza viruses, obtained from clinical material collected in Austria, were analyzed by sequencing the NA region as indicated in Table 1. Among these samples, a CAT-to-TAT codon change, leading to the oseltamivir resistance mutation H275Y, was found in the 12 seasonal A(H1N1) reference strains and in none of the remaining 153 seasonal A(H1N1) viruses. The H275Y mutation was also found in three of the 220 A(H1N1)2009 viruses. In addition, one oseltamivir-sensitive A(H1N1)2009 virus showed a N272D mutation, which is located within the probe region of the NA-specific PCR. No GAG-to-GTG codon change, resulting in the E119V mutation, was found in the 49 A(H3N2) viruses investigated. The two resistant A(H1N1)2009 reference strains showed the H275Y and the two resistant A(H3N2) reference strains showed the E119V mutation coding for oseltamivir resistance. An overview on the influenza virus strains analyzed by sequencing during the three seasons and those selected for the evaluation of MPA is provided in Table 3.
Detection of H275Y and E119V point mutations by MPA
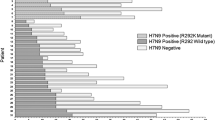
Figure 1 gives an overview on the melting curve data for selected individual strains. A melting point (Tm) value of 55.2 ± 0.3°C was obtained for all 42 of the oseltamivir-sensitive seasonal A(H1N1) strains tested, whereas a much lower Tm value of 44.2 ± 0.5°C was obtained for all 12 of the oseltamivir-resistant variants carrying the H275Y mutation. Likewise, a Tm value of 53.2 ± 0.7°C was obtained with 49 sensitive A(H3N2) strains and the lower Tm value of 45.8 ± 0.7°C with the two resistant A(H3N2) reference strains showing the E119V mutation. The two resistant A(H1N1)2009 reference strains and the three resistant A(H1N1)2009 strains obtained during season 2010/11 had its Tm at 44.0 ± 0.3°C and 216 of the 217 sensitive A(H1N1)2009 strains had their Tm at 54.7 ± 0.3°C. The oseltamivir-sensitive A(H1N1)2009 strain, which had the point mutation N272D within the probe-binding region of the PCR product, had its melting temperature at 40.8°C. The sensitivity to oseltamivir of this strain was also confirmed by functional NI resistance testing. Information on the different Tms and their standard deviations for resistant and sensitive strains for all three influenza subtypes are summarized in Table 3.
Representative melting curves with resistant and sensitive seasonal A(H1N1), A(H1N1)2009, and A(H3N2) viruses. The x-axis shows the temperature in °C and the y-axis shows the fluorescence data expressed as the negative of the first derivative of the fluorescence intensity over time (−d/dT). The strong melting peak at 80°C corresponds to the melting points of the whole amplicon and primer dimer products, and is detected in addition to the melting peaks for the amplicon–probe hybrids. A missing peak at 80°C leads to an invalid result
For the interpretation of the MPA test results, a result is valid if a strong melting peak at 80°C, corresponding to the melting points of the whole amplicon and primer dimer products, is detected in addition to the melting peaks for the amplicon–probe hybrids. A missing peak at 80°C leads to an invalid result.
Antiviral resistance testing by MPA revealed identical results to those obtained by sequencing the NA gene for all 54 A(H1N1), 222 A(H1N1)2009, and 51 A(H3N2) samples tested by both methods.
The reproducibility of the MPA was assessed by the determination of the intra- and inter-assay variability. The intra-assay variability testing (sensitive and resistant strain of each subtype tested 10 times within the same run) revealed a mean Tm (SD) for resistant seasonal A(H1N1), A(H1N1)2009, and A(H3N2) strains of 43.9°C (SD 0.2), 44.1°C (SD 0.2), and 46.3°C (SD 0.1), respectively, and for the sensitive strains of 55.2°C (SD 0.1), 54.9°C (SD 0.3), and 53.5°C (SD <0.1). The inter-assay variability was determined by repeated testing of oseltamivir-resistant and -sensitive viruses of all three subtypes in 18 different runs. The results obtained for the three different assays are provided in Table 3 and demonstrate the high reproducibility of MPA.
To investigate whether oseltamivir-sensitive and -resistant mutant strains can be discriminated in mixed viral populations, resistant and sensitive tissue-culture-derived stock virus preparations were mixed in defined proportions ranging from 95% wild-type strain to 95% mutant strain and analyzed using the MPA procedure described above. The lowest proportion of resistant viruses detectable in these mixtures was 10% for seasonal A(H1N1), A(H1N1)2009, and A(H3N2) mutant viruses (Table 4). Examples of melting point profiles of mixed viral populations (oseltamivir-resistant and -sensitive strains) are shown in Fig. 2.
Examples of melting point profiles of mixed viral populations (oseltamivir-resistant and -sensitive strains): a melting point profiles of A(H3N2) mixtures containing 80% oseltamivir-sensitive and 20% oseltamivir-resistant strains (blue) and 80% oseltamivir-resistant and 20% oseltamivir-sensitive strains (red); b melting point profiles of five A(H1N1)2009 mixtures containing the following percentages of resistant:sensitive strains: (1) 90:10, (2) 80:20, (3) 50:50, (4) 30:70, (5) 20:80
Comparison of the MPA of influenza A virus strains from nasopharyngeal swabs and cell culture isolates
Because of the need for early information on the drug resistance profile of influenza viruses (for example, in immunocompromised patients), we investigated retrospectively whether MPA with influenza viruses derived directly from clinical material is feasible and provides reliable data. Therefore, the results obtained by MPA using viruses isolated by two to three passages on MDCK cells (= isolates) were compared with those obtained by MPA using their corresponding clinical material. Identical results were obtained for all 25 A(H1N1) viruses (7 oseltamivir-resistant and 18 sensitive). The mean ct-value of the initially performed diagnostic PCR of these clinical materials was 30.3 (SD 4.5) and 21.6 (SD 4.9) for the corresponding isolates. Identical results were also obtained for the 44 sensitive A(H3N2) viruses. These clinical samples had a mean ct-value in the diagnostic PCR of 30.6 (SD 3.1) and of 22.6 (SD 1.7) for the isolates. Attempts to perform MPA directly from clinical material was not possible with 3 out of 30 A(H1N1)2009-positive samples (10%), most probably due to the low viral load in the retrospectively analyzed portions of these clinical materials. The mean ct-value of the initially performed diagnostic PCR of these three clinical samples was 38.8 (SD 2.1). For the remaining 27 A(H1N1)2009 clinical samples, where MPA was possible, the mean ct-value of the diagnostic PCR was 33.5 (SD 3.2) and 29.3 (SD 2.0) for their isolates.
All of the MPA results obtained from the clinical material were identical to those obtained by sequencing the NA gene of the corresponding isolates.
Discussion
The high mutation rate of influenza viruses, combined with the increasing worldwide use of influenza-virus-specific drugs, allows the selection of viruses that are resistant to the currently available antiviral medications. Therefore, a widely available and reliable test for the rapid detection of drug-resistant influenza virus strains, both for the purpose of influenza surveillance programs and for making decisions concerning the treatment of patients with an increased risk for severe influenza disease, is required. One way to obtain results on antiviral susceptibility more rapidly is to use pyrosequencing [23], but equipment for pyrosequencing is expensive and is currently not part of the standard equipment of most laboratories. Also, a reverse transcription PCR coupled with a restriction fragment length polymorphism assay has been described for seasonal A(H1N1) viruses, but it is a lengthy process and not practical for high-volume testing [24]. Another study describes a multiplex reverse transcription PCR for the subtyping and determination of oseltamivir resistance for seasonal and pandemic A(H1N1) viruses using multiplex PCR on a Luminex platform [25], but, again, this technique depends on the availability of specific platforms. Alternatives to obtain quick information on antiviral susceptibility are reverse transcription PCR assays using SNP probes [26–30]. The results of these studies demonstrate a quick and reliable discrimination of the H275Y mutation either for seasonal or pandemic influenza A(H1N1) viruses. Nevertheless, due to the multiplex format of these assays, their laboratory setup is complex and their sensitivities are sometimes lower compared to uniplex PCRs.
The aim of our study was to establish MPA as an easy and rapid method for the detection of mutations leading to oseltamivir resistance. Of the many resistant mutations reported [31], we primarily focused on those most commonly observed. Nevertheless, the assay protocol can easily be adapted to detect any of the other mutations associated with neuraminidase inhibitor resistance. In addition, the results of the present study clearly demonstrate that MPA is a highly reproducible and very sensitive method, allowing the discrimination of resistant mutant strains in mixtures of oseltamivir-resistant and -sensitive viral populations. Although MPA does not give information of the quantity of resistant virus in the mixture, we could show that it is possible to detect resistant mutant strains of influenza viruses in the presence of sensitive strains, even when they represented only 10% of the population. This clearly shows that the reliable detection of evolving resistant virus variants in patients undergoing treatment is possible.
In addition, the results obtained by MPA performed with virus RNA extracted directly from clinical material demonstrate that, by using this rapid and simple method, results comparable to those obtained by sequencing can be obtained within 5 h, which is similar to the time required for pyrosequencing. Shorter turn-around times can be achieved using one-step reverse transcription real-time PCR using SNP probes. The possible disadvantage of this multiplex methodology is its complex implementation and a sometimes decreased sensitivity compared to uniplex PCRs.
The theoretical problem that any mutations within the probe region lead to a decrease of the Tm was clearly disapproved by the results obtained with the one strain carrying the N272D mutation within the probe region. The different Tms of the H275Y mutation and the N272D mutation clearly indicate that, in this system, the melting point is characteristic of a particular point mutation, enabling substitutions associated with antiviral resistance to be clearly identified.
In summary, MPA has the advantage that it can be implemented easily for routine diagnostics by laboratories that perform real-time PCR and, therefore, provides a fast and widely available tool for clinical decisions on antiviral medication, especially in cases where neuraminidase inhibitor resistance is suspected. In addition, it represents a relatively cost-effective method for the large-scale screening of single-point mutations on widely used PCR platforms, and, by developing additional protocols, the detection of any mutation in the NA gene associated with NI resistance is possible.
References
Chiu SS, Lau YL, Chan KH, Wong WH, Peiris JS (2002) Influenza-related hospitalizations among children in Hong Kong. N Engl J Med 347(26):2097–2103. doi:10.1056/NEJMoa020546
Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA (1997) Influenza virus infections in infants. Pediatr Infect Dis J 16(11):1065–1068
Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K (2000) Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 342(4):232–239. doi:10.1056/NEJM200001273420402
Sullivan KM, Monto AS, Longini IM Jr (1993) Estimates of the US health impact of influenza. Am J Public Health 83(12):1712–1716
World Health Organization (WHO) (2005) Influenza fact sheet
Gubareva LV, Kaiser L, Matrosovich MN, Soo-Hoo Y, Hayden FG (2001) Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J Infect Dis 183(4):523–531. doi:10.1086/318537
Hatakeyama S, Sugaya N, Ito M, Yamazaki M, Ichikawa M, Kimura K, Kiso M, Shimizu H, Kawakami C, Koike K, Mitamura K, Kawaoka Y (2007) Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA 297(13):1435–1442. doi:10.1001/jama.297.13.1435
Kawai N, Ikematsu H, Iwaki N, Kawashima T, Maeda T, Mitsuoka S, Kondou K, Satoh I, Miyachi K, Yamaga S, Shigematsu T, Hirotsu N, Kashiwagi S (2007) Longer virus shedding in influenza B than in influenza A among outpatients treated with oseltamivir. J Infect 55(3):267–272. doi:10.1016/j.jinf.2007.05.176
Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y (2004) Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364(9436):759–765. doi:10.1016/S0140-6736(04)16934-1
Stephenson I, Democratis J, Lackenby A, McNally T, Smith J, Pareek M, Ellis J, Bermingham A, Nicholson K, Zambon M (2009) Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin Infect Dis 48(4):389–396. doi:10.1086/596311
Hauge SH, Dudman S, Borgen K, Lackenby A, Hungnes O (2009) Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007–08. Emerg Infect Dis 15(2):155–162
Lackenby A, Hungnes O, Dudman SG, Meijer A, Paget WJ, Hay AJ, Zambon MC (2008) Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill 13(5):pii:8026
Wong SS, Yuen KY (2008) Antiviral therapy for respiratory tract infections. Respirology 13(7):950–971. doi:10.1111/j.1440-1843.2008.01404.x
Gubareva LV (2004) Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res 103(1–2):199–203. doi:10.1016/j.virusres.2004.02.034
Tisdale M (2000) Monitoring of viral susceptibility: new challenges with the development of influenza NA inhibitors. Rev Med Virol 10(1):45–55. doi:10.1002/(SICI)1099-1654(200001/02)10:1<45::AID-RMV265>3.0.CO;2-R
Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Tashiro M, Webster RG, Aymard M, Hayden FG, Zambon M (2006) Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother 50(7):2395–2402. doi:10.1128/AAC.01339-05
Wetherall NT, Trivedi T, Zeller J, Hodges-Savola C, McKimm-Breschkin JL, Zambon M, Hayden FG (2003) Evaluation of neuraminidase enzyme assays using different substrates to measure susceptibility of influenza virus clinical isolates to neuraminidase inhibitors: report of the neuraminidase inhibitor susceptibility network. J Clin Microbiol 41(2):742–750
Göhring K, Mikeler E, Jahn G, Hamprecht K (2006) Rapid simultaneous detection by real-time PCR of cytomegalovirus UL97 mutations in codons 460 and 520 conferring ganciclovir resistance. J Clin Microbiol 44(12):4541–4544. doi:10.1128/JCM.01141-06
Redlberger M, Aberle SW, Heinz FX, Popow-Kraupp T (2007) Dynamics of antigenic and genetic changes in the hemagglutinins of influenza A/H3N2 viruses of three consecutive seasons (2002/2003 to 2004/2005) in Austria. Vaccine 25(32):6061–6069. doi:10.1016/j.vaccine.2007.05.045
Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC (2004) Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 42(4):1564–1569
Overduin P, Jenny S, Meijer A (2011) Influenza A PCR Light Cycler - probe test A-matrix-B-NS-H1-H1v-H3-H5-N1-N1v-N1avian-N2. RIVM Laboratory Protocol Library document; https://extranet.ecdc.europa.eu/EISN/Shared%20Documents/Document%20library%20and%20past%20meetings/CNRL%20library/Laboratory%20Protocol%20Library/Molecular%20Detection/Influenza_diagnostic_qPCR_RIVM_2011.pdf
World Health Organization (WHO) (2010) WHO External Quality Assessment Programme for the Detection of Influenza Virus Type A by PCR, Summary Report Panel 7 (January–March 2010)
Duwe S, Schweiger B (2008) A new and rapid genotypic assay for the detection of neuraminidase inhibitor resistant influenza A viruses of subtype H1N1, H3N2, and H5N1. J Virol Methods 153(2):134–141. doi:10.1016/j.jviromet.2008.07.017
Guo L, Garten RJ, Foust AS, Sessions WM, Okomo-Adhiambo M, Gubareva LV, Klimov AI, Xu X (2009) Rapid identification of oseltamivir-resistant influenza A(H1N1) viruses with H274Y mutation by RT-PCR/restriction fragment length polymorphism assay. Antiviral Res 82(1):29–33. doi:10.1016/j.antiviral.2009.01.004
Mahony JB, Chong S, Luinstra K, Petrich A, Smieja M (2010) Development of a novel bead-based multiplex PCR assay for combined subtyping and oseltamivir resistance genotyping (H275Y) of seasonal and pandemic H1N1 influenza A viruses. J Clin Virol 49(4):277–282. doi:10.1016/j.jcv.2010.08.006
Wong S, Pabbaraju K, Wong A, Fonseca K, Drews SJ (2011) Development of a real-time RT-PCR assay for detection of resistance to oseltamivir in influenza A pandemic (H1N1) 2009 virus using single nucleotide polymorphism probes. J Virol Methods 173(2):259–265. doi:10.1016/j.jviromet.2011.02.014
Bolotin S, Robertson AV, Eshaghi A, De Lima C, Lombos E, Chong-King E, Burton L, Mazzulli T, Drews SJ (2009) Development of a novel real-time reverse-transcriptase PCR method for the detection of H275Y positive influenza A H1N1 isolates. J Virol Methods 158(1–2):190–194. doi:10.1016/j.jviromet.2009.01.016
Carr MJ, Sayre N, Duffy M, Connell J, Hall WW (2008) Rapid molecular detection of the H275Y oseltamivir resistance gene mutation in circulating influenza A (H1N1) viruses. J Virol Methods 153(2):257–262. doi:10.1016/j.jviromet.2008.07.011
Operario DJ, Moser MJ, St George K (2010) Highly sensitive and quantitative detection of the H274Y oseltamivir resistance mutation in seasonal A/H1N1 influenza virus. J Clin Microbiol 48(10):3517–3524. doi:10.1128/JCM.01031-10
van der Vries E, Jonges M, Herfst S, Maaskant J, Van der Linden A, Guldemeester J, Aron GI, Bestebroer TM, Koopmans M, Meijer A, Fouchier RA, Osterhaus AD, Boucher CA, Schutten M (2010) Evaluation of a rapid molecular algorithm for detection of pandemic influenza A (H1N1) 2009 virus and screening for a key oseltamivir resistance (H275Y) substitution in neuraminidase. J Clin Virol 47(1):34–37. doi:10.1016/j.jcv.2009.09.030
Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, Bright RA, Butler EN, Wallis TR, Klimov AI, Gubareva LV (2008) Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother 52(9):3284–3292. doi:10.1128/AAC.00555-08
Acknowledgments
The authors thank Daniela Schmidt and Barbara Dalmatiner for their excellent technical assistance.
Transparency declaration
The authors declare no conflict of interest of any nature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Redlberger-Fritz, M., Aberle, S.W., Strassl, R. et al. Rapid identification of neuraminidase inhibitor resistance mutations in seasonal influenza virus A(H1N1), A(H1N1)2009, and A(H3N2) subtypes by melting point analysis. Eur J Clin Microbiol Infect Dis 31, 1593–1601 (2012). https://doi.org/10.1007/s10096-011-1482-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1482-9