Abstract
In 2006, our clinical microbiology laboratory suspected that our institution was experiencing an increase in Acinetobacter baumannii infections and was concerned about resistance. A cross-sectional study was conducted to determine the A. baumannii antibiogram for 2006 and assess the appropriateness of antibiotics therapy. The study included all adult inpatients with a positive culture for A. baumannii between January 1 2006 and December 31 2006. A total of 129 isolates were identified. A. baumannii was highly susceptible to imipenem (97.7%) and meropenem (95.3%). Among the aminoglycosides, A. baumannii had reduced susceptibility to gentamicin (40.5%). Based on their susceptibility patterns, only 76 (58.9%) antibiotics regimens were susceptible against the isolates. At our institution, A. baumannii remains highly susceptible to the carbapenems and aminoglycosides. We encourage our practitioners to analyze the susceptibility pattern of each isolate when ordering antibiotics, which will help increase our rate of appropriate antibiotic selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter baumannii is a gram-negative bacilli that can survive for prolonged periods in the environment and cause urinary tract infections, skin and soft-tissue infections, pneumonia, bacteremia, meningitis, and osteomyelitis. This organism has emerged as a significant nosocomial pathogen in hospitalized patients worldwide and is associated with high mortality rates [1–3]. The SCOPE surveillance study, a large prospective study of nosocomial bloodstream infections (BSIs) conducted in the United States from 2000–2001, ranked A. baumannii as the tenth leading cause of BSIs in the country (0.6 BSIs per 10,000 admissions; 1.3% of all monomicrobial BSIs). However, A. baumannii ranked third in crude mortality (34.0%), behind only Candida sp. (39.2%) and Pseudomonas aeruginosa (38.7%) [4]. A. baumannii is also a common cause of late-onset ventilator-associated pneumonia (>5 days after admission) and is associated with a higher mortality (30–75%) than other bacteria [5–7]. The National Nosocomial Infections Surveillance System determined the epidemiology of gram-negative bacilli in intensive care units (ICUs) in the United States for the most common types of nosocomial infections during the period 1986–2003. Acinetobacter species ranked fourth (6.9%) in prevalence among gram-negative organisms causing pneumonia in ICUs. However, it was the only gram-negative organism with a significant increase in prevalence from 1986 to 2003 [8].
Multidrug-resistant (MDR) infections are a growing problem and have been reported worldwide. A. baumannii has been become increasingly difficult to treat because of the emergence of strains resistant to all drugs or all but one commonly prescribed antibiotics. These MDR strains are sometimes only susceptible to polymyxins (colistin and polymyxin B), a class of antibiotics that has not been used commonly for decades and is more toxic than the commonly prescribed antibiotics [1–2, 9, 10].
Our clinical microbiology laboratory suspected that our institution was experiencing an increase in A. baumannii infections and was concerned about resistance. A cross-sectional study was conducted at our institution with two objectives: first, to determine the A. baumannii antibiogram for 2006, and second, to assess the appropriateness of antibiotic therapy for A. baumannii infections.
Methods
A cross-sectional study was conducted using electronic pharmacy and microbiology laboratory patient data from January 1 2006 through December 31 2006 from a tertiary care hospital in Augusta, Georgia. A computer-generated list was created from PathNet (Cerner, Kansas City, MO, USA) to identify all patients by medical record number (MRN) with an A. baumannii isolate. From the list of MRNs, a database was created from the microbiology laboratory data, which included the MRN, date of admission, site of isolate, date of collection, and date of final verification. Next, a computer-generated database was created from PharmNet (Cerner, Kansas City, MO, USA) to gather the antibiotic data for the same list of patients identified in PathNet.
An isolate was included in the final database if it was the first A. baumannii isolate per site of infection per admission. Duplicate isolates were counted if they were collected >14 days after the previous isolate during the same admission. Isolates were from all adult (age ≥13 years) inpatient admissions with a positive culture for A. baumannii. Isolates were collected from both sterile and nonsterile sites. In addition, patient demographic data, including age, gender, and hospital unit, were collected. Infections were considered to be nosocomial if the isolate was collected >48 h after hospital admission.
Antibiotics susceptibility testing
Minimum inhibitory concentrations (MICs) were determined using a commercial broth microdilution (MicroScan negative MIC type 30 panel on a WalkAway instrument, Dade Behring, West Sacramento, CA, USA). Testing was conducted for the following antibiotics: amikacin, ampicillin-sulbactam, cefepime, ceftazidime, ciprofloxacin, gentamicin, imipenem, levofloxacin, meropenem, tetracycline, ticarcillin-clavulanate, tobramycin, and trimethoprim-sulfamethoxazole.
In this study, we reported MDR as nonsusceptibility, which was defined as the sum of the intermediate and resistant isolates. Antibiotic selection was considered to be inappropriate if the infection was not being effectively treated at the time when both the A. baumannii isolate was identified and its antibiotic susceptibility pattern was reported.
Data analysis
The two databases were linked electronically by the patient’s MRN in Access (Microsoft, Redmond, WA, USA). The data was analyzed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
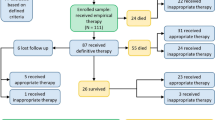
A total of 129 isolates were identified using the inclusion criteria from January 1 2006 through December 31 2006. Table 1 lists the sites of infection. Eighty-one (62.8%) isolates were from respiratory cultures. A respiratory culture was defined as an isolate collected via bronchoalveolar lavage, nasopharyngeal aspirate, sputum, throat swab, and tracheostomy aspirate. The mean time to final culture verification was 6.1 days.
The isolates came from 80 patients, who had a combined total of 86 admissions. The mean length of stay for the 86 admissions was 28.6 days. Sixty of the 86 (69.8%) admissions included one day or more in the ICU. Sixty-two (72.1%) of the 86 admissions contained an isolate that was classified as a nosocomial infection.
Table 2 shows the institution’s antibiogram for A. baumannii in 2006. A. baumannii was highly susceptible to imipenem (97.7%) and meropenem (95.3%). Among the aminoglycosides, A. baumannii was highly susceptible to amikacin (93.6%) and tobramycin (92.3%), but had reduced susceptibility to gentamicin (40.5%). The isolates were found to have reduced susceptibility to ampicillin-sulbactam (46.5%) and tetracycline (41.4%).
Multidrug nonsusceptibility
Only 35 (27.1%%) isolates were susceptible to all thirteen of the tested antibiotics. Six (4.7%) isolates were nonsusceptible to only one antibiotic. Eighty-eight (68.2%) isolates were nonsusceptible to three or more antibiotics. No isolates were nonsusceptible to all thirteen antibiotics (Table 3).
Appropriate antibiotic selection
One hundred twenty-nine antibiotic regimen were identified from the pharmacy database. Out of the 129 regimens, 76 (58.9%) regimens were susceptible against the isolates based on their susceptibility patterns. Of 53 regimens that were nonsusceptible, the most common antibiotics ordered were levofloxacin [34 (64.2%)], cefepime [6 (11.3%)], ampicillin-sulbactam [4 (7.6%)], and gentamicin [4 (7.6%)].
A total of 67 regimens were identified when controlling for empiric use. Empiric regimens were defined as regimens that were administered for 6 days or less. This definition was based on the mean time to final culture verification (6.1 days) identified in the study. Out of the 67 regimens, 41 (61.2%) regimens were susceptible against the isolates based on their in vitro susceptibility patterns. Of 26 regimens that were nonsusceptible, the most common antibiotics administered were levofloxacin [20 (76.9%)] and cefepime [3 (11.5%)].
Discussion
Overall, the A. baumannii isolates at our institution were highly susceptible to carbapenems, with 97.7% of the isolates susceptible to imipenem and 95.3% susceptible to meropenem. The potential for the emergence of carbapenem resistance is a concern because A. baumannii isolates are frequently resistant to aminoglycosides, fluoroquinolones, and third-generation cephalosporins. Recent epidemiologic studies from around the world have reported A. baumannii isolates that are resistant to carbapenems [11–13].
We also found the isolates to be susceptible in vitro to two aminoglycosides, amikacin (93.6%) and tobramycin (92.3%). Gentamicin had reduced susceptibility (40.5%) for unknown reasons. Despite the high susceptibility to carbapenems and aminoglycosides, we found 88 (68.2%) isolates that were nonsusceptible to three or more of the antibiotics tested.
Antibiotic selection was only appropriate in 58.9% of the regimens and 61.2% of the regimens when adjusting for empiric use (regimens of duration >6 days). Using inappropriate antibiotics can result in treatment failures. These results emphasize the importance of monitoring cultures and their susceptibilities. Ibrahim et al. reported a reduction in fatality in patients receiving appropriate antibiotics regimens (61.9% vs. 28.4%, RR=2.18) [14]. Zaragoza et al. conducted a study on inadequate empirical antibiotics treatment in ICU patients with BSIs. The study reported that 23.5% of patients with a BSI received inappropriate empiric antibiotics treatment. The study failed to demonstrate a relationship between mortality and the administration of inappropriate empiric antibiotics in ICU patients with bacteremia [15]. However, inappropriate therapy can lead to increased morbidity, medication cost, length of stay, and, potentially, mortality.
The limitations of the present study include its study design, possible concurrent infections, and not obtaining the data by diagnosis-related groups (DRG). A cross-sectional study design only provides descriptive statistics. However, cross-sectional studies do provide information about the topic for the specified period of time. We obtained all data electronically. By conducting a retrospective chart review, we could have gathered data on mortality, severity of disease, colonization, and concurrent infections. Our percentage of appropriate antibiotic selection may have improved if we could have proved that some antibiotics were being used for a separate infection. Lastly, we do not know if these patients were primarily in the hospital for an A. baumannii infection. Obtaining the data by DRG would have helped clarify this. Our mean length of stay was 28.6 days. Part of the length stay could be due to other diseases, which could be identified by a DRG.
Our study found a high prevalence of MDR A. baumannii at our institution. However, A. baumannii remains highly susceptible to the carbapenems and aminoglycosides. We encourage our practitioners to analyze the susceptibility pattern of each isolate when ordering an antibiotic regimen, which will help increase our rate of appropriate antibiotic selection.
References
Sunenshine RH, Wright MO, Maragakis LL et al (2007) Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13:97–103
Jain R, Danziger LH (2004) Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother 38:1449–1459
Levin AS, Levy CE, Manrique AEI et al (2003) Severe nosocomial infections with imipenem-resistant Acinetobacter baumannii treated with ampicillin/sulbactam. Int J Antimicrob Agents 21:58–62
Wisplinghoff H, Bischoff T, Tallent SM et al (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317
Wood CG, Hanes SD, Croce MA et al (2002) Comparison of ampicillin-sulbactam and imipenem-cilastatin for the treatment of Acinetobacter ventilator-associated pneumonia. Clin Infect Dis 34:1425–1430
Bergogne-Bérézin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev 9:148–165
Jellison TK, McKinnon PS, Rybak MJ (2001) Epidemiology, resistance, and outcomes of Acinetobacter baumannii bacteremia treated with imipenem-cilastatin or ampicillin-sulbactam. Pharmacotherapy 21:142–148
Gaynes R, Edwards JR; National Nosocomial Infections Surveillance System (2005) Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41:848–854
Holloway KP, Rouphael NG, Wells JB et al (2006) Polymyxin B and doxycycline use in patients with multidrug-resistant Acinetobacter baumannii infections in the intensive care unit. Ann Pharmacother 40:1939–1945
Rahal JJ (2006) Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis 43:S95–S99
Manikal VM, Landman D, Saurina G et al (2000) Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis 31:101–106
Mahgoub S, Ahmed J, Glatt AE (2002) Completely resistant Acinetobacter baumannii strains. Infect Control Hosp Epidemiol 23:477–479
Hsueh PR, Teng LJ, Chen CY et al (2002) Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg Infect Dis 8:827–832
Ibrahim EH, Sherman G, Ward S et al (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155
Zaragoza R, Artero A, Camarena JJ et al (2003) The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin Microbiol Infect 9:412–418
Acknowledgments
The authors gratefully acknowledge the assistance of Deanna Craig and Susan Jones in extracting the microbiology data presented. This study received no funding. We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dauner, D.G., May, J.R. & Steele, J.C.H. Assessing antibiotic therapy for Acinetobacter baumannii infections in an academic medical center. Eur J Clin Microbiol Infect Dis 27, 1021–1024 (2008). https://doi.org/10.1007/s10096-008-0537-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0537-z