Abstract
The purpose of this study was to assess the one-year efficacy of highly active antiretroviral therapy (HAART) administered by general practitioners in a primary care community clinic in rural South Africa. We performed an observational cohort study of 675 treatment-naïve human immunodeficiency virus (HIV)-infected patients (including 66 children) who began HAART at least 12 months prior to the data analyses. Throughout treatment, the CD4+ T-cell count (percentage of CD4+ T-cells in children) and plasma HIV-RNA level were determined and the patient’s weight was recorded. The primary outcome was mortality. Secondary outcomes were viral suppression, immunological response, and weight gain. One year after the start of HAART, 100 of the 675 (15%) patients were lost to follow-up and 119 patients (18%), including six children, died. Mortality was highest during the first few months of treatment. Based on an on-treatment analysis at one year after the start of therapy, 83% of adults and 71% of children had a viral load <400 copies/ml; the viral load was <50 copies/ml in 70% of adults and 61% of children. At one year, the mean CD4+ T-cell count in adults had increased by 236/mm3, and the mean body mass index (BMI) had increased by 3.5 kg/m2. In children, the mean CD4% had increased by 17.6. A low Karnofsky score and a low baseline CD4+ T-cell count were independently associated with death. In addition to these factors, a low baseline BMI and gender were predictive of a poor immunological outcome. Our study shows that adequately monitored HIV/acquired immunodeficiency syndrome (AIDS) care administered by general practitioners and their staff is feasible and leads to good results in a rural, primary care center in sub-Saharan Africa. In order to achieve even better results, early mortality should be reduced and efforts should be made to start HAART earlier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly active antiretroviral therapy (HAART) is acknowledged worldwide as the standard of care for people with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) [1–3]. It decreases plasma HIV-RNA levels, increases CD4+ T-cell counts, decreases morbidity, and prolongs survival in HIV-infected patients [4, 5]. Several studies have shown that HAART can be applied with the same efficacy in developing and developed countries [6–11]. However, concerns about the lack of infrastructure, irregular medication supply, and poor adherence has led to pessimism about the feasibility of HAART programs in resource-constrained settings. Figures from the World Health Organization (WHO) show that only 17% of people who need HAART in sub-Saharan Africa actually receive it [12]. The failure of initial antiretroviral regimens and the emergence of widespread antiretroviral drug resistance, which would reduce the long-term durability of HAART in developing countries, is a serious concern.
The implementation of HAART in rural areas of resource-limited countries can be hazardous. This observational study reports on the one-year efficacy of HAART in a sub-Saharan, rural setting in 675 consecutive patients who were initiated antiretroviral therapy from September 2003 through April 2006. The patients were treated in a primary care setting and the treatment was administered and monitored by general practitioners. In addition to clinical and immunological data, longitudinal viral load testing was also performed.
Methods
Site and patients
Elandsdoorn is a township with an estimated population of 40,000. It is situated in a poor, rural area in Mpumalanga, a province in the northeast of the Republic of South Africa. AIDS is the leading cause of death in adults in South Africa, and it is estimated that about 48% of deaths are related to HIV [13]. In a Mpumalanga-based study, the HIV prevalence among adults aged 15 to 49 years was 23.1% [14]. Among antenatal clinic attendees, HIV prevalence was 30.8% [15].
The Ndlovu Medical Centre is a non-governmental organization in Elandsdoorn (http://www.elandsdoorn.com/) that provides “paid for service” primary health care, as well as prevention-, tuberculosis-, and HIV/AIDS-programs which are donor-funded and free of charge for the patient.
During the study period, five general practitioners were in charge of running the outpatient clinic. The clinic has a maternity ward with 16 beds. Some of these beds can also be used for HIV/AIDS patients. The clinic is equipped with X-ray and ultrasound equipment, a pharmacy, and a laboratory with facilities to perform CD4+ T-cell counts, plasma HIV-RNA analysis, full blood counts, and blood chemistry investigations. In 2003, a privately subsidized program for the provision of HAART was initiated.
In accordance with the WHO guidelines, adults were eligible for antiretroviral therapy if they had a WHO stage IV AIDS-defining illness, irrespective of their CD4+ T-cell count, or if their CD4+ T-cell count was below 200/mm3, irrespective of their clinical stage [1, 2]. Children were eligible if they had a WHO stage C disease or a CD4 percentage of less than 15 [1, 3].
Patients could enroll in the program if they lived within a 25-km radius of the clinic. To optimize adherence, a number of psychosocial conditions were established for inclusion: (1) disclosure of HIV status to at least one relative or friend who had to accompany the patient to the clinic when he/she came for information and counseling; (2) a relative or friend willing to support the patient in treatment adherence (family and friends assisted treatment adherence; FAST); and (3) demonstrated reliability, i.e., the patient had attended three or more scheduled visits to the clinic over a three-month period. The final decision to treat was ascertained by a multidisciplinary team including both community members and the caregiver. During HAART, the patients were seen by a doctor and counselor every two weeks for the first two months and monthly thereafter. The counselors completed questionnaires on employment, means of income, education, and household structures when the patients entered the program.
Antiretroviral therapy was in accordance with the national guidelines [16]. Unless contraindicated, all patients (children and adults) started therapy with stavudine (d4T, 40 mg bid or 30 mg bid if their weight was less than 60 kg), lamivudine (3TC, 150 mg bid), and efavirenz (EFV, 600 mg qd) or nevirapin (NVP, 200 mg bid). All drugs were administered separately, as triomune was not available. Later, during the inclusion period, patients often began receiving Combivir combined with either nevirapin or efavirenz. Young children were given syrups and older children were given tablets. The dosage depended on the child’s weight.
For second-line treatment, non-nucleoside reverse transcriptase inhibitors (NNRTIs) could be switched to the protease inhibitors (PIs) lopinavir/ritonavir. The efavirenz dose was not adjusted during treatment with rifampin. HIV-infected patients with pulmonary tuberculosis and a CD4+ T-cell count higher than 200 /mm3 were treated for tuberculosis, and antiretroviral therapy was deferred until they met the WHO criteria. In patients with tuberculosis and a CD4+ T-cell count of less than 200/mm3, antiretroviral therapy was initiated after the completion of two months of tuberculosis therapy or, simultaneously, depending on the clinical status of the patient.
The counselors monitored adherence to HAART during the patients’ visits to the clinic and during home visits. If necessary, the patients received funds to cover the cost of transport to the clinic and food parcels.
Data collection
Data were retrospectively collected from the medical charts. Patients entered the program from September 2003 through April 2006, by definition, at least 12 months before the data analyses, and were all treatment-naïve when starting HAART. The clinic also treats pregnant women according to the prevention of mother-to-child transmission (pMTCT) protocol. Data from the pMTCT program were not included in this study, as inclusion of the pMTCT recipients would make the study population more heterogeneous.
Adults’ body mass indices (BMI, weight/height2) and children’s weights were recorded during each visit. The Karnofsky performance score and the WHO staging system were used to establish the patient’s clinical condition when HAART was initiated. At baseline, the CD4+ T-cell count (FACSCount system, Becton Dickinson Biosciences, San Jose, CA), the plasma HIV-RNA level (System 340 bDNA analyzer, Bayer AG, Leverkusen, Germany), and a full blood count were measured, and at 6, 12, 24, 36, and 52 weeks, the plasma HIV-RNA level, CD4+ T-cell count (CD4% in children), full blood count, and alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinin, urea, and amylase concentrations were measured.
Statistical analysis
The data were analyzed on both an intention-to-treat (ITT) and an on-treatment (OT) basis. The primary endpoint was mortality from all causes during the first year after starting HAART. Secondary endpoints were the proportion of patients with a viral load of less than 50 and less than 400 copies/ml, good immunological response as defined by a CD4+ T-cell count higher than 200/mm3 or a CD4% of 15 or higher in children, and an increase in BMI or weight. We ignored treatment changes and interruptions.
To define predictors of treatment efficacy, we compared the outcome (survival and immunological response) of adult patients who presented a poor clinical condition at baseline, as defined by a BMI in the lowest quartile, a Karnofsky score of 50 or less, and a CD4+ T-cell count of 50/mm3 or less, with that of patients who were in better condition.
Continuous data were compared with Student’s t-test or the paired t-test, as appropriate. Proportions were compared with the χ2 test, and data that were not normally distributed were analyzed via the Mann-Whitney U-test or Wilcoxon’s test. Kaplan-Meier survival analyses were used to estimate the time from the initiation of antiretroviral therapy to viral suppression and good immunologic response. Odds ratios were calculated using logistic regression analysis. The Cox proportional-hazards model was used to identify independent predictors of the endpoints. To this end, a multivariate analysis was performed for all variables that were significant (p<0.10) in the univariate analysis.
Results
Patient characteristics
Between September 2003 and May 2006, the Ndlovu Medical Center provided HIV voluntary counseling and testing (VCT) to 5,226 patients, of whom 2,442 (47%) were tested as HIV seropositive (Fig. 1). Of these, 675 (28%) started antiretroviral therapy and were included in our analysis; 609 (90%) were adults (age range 16 to 73 years) and 66 (10%) were children (age range eight months to 11 years). Fifty-four percent of the adults and 77% of the children were also treated for tuberculosis.
The baseline patient characteristics and initial antiretroviral therapy regimens of the adults and children are summarized in Table 1. At presentation, 32 patients (5%) were too weak to stand and a BMI could not be calculated. In the remaining patients, the median BMI was significantly lower in men (18.6 kg/m2) than in women (20.3 kg/m2, p<0.01). There was also a significant difference in the median CD4+ T-cell counts between men and women of 54/mm3 and 74/mm3, respectively (p=0.02). The median weight of the children at baseline was 13.8 kg. The low average socio-economic status is shown by the high unemployment rate among participants and widespread dependency on government grants.
Patient outcome
At 12 months after HAART initiation, 407 of 609 adults (67%) and 49 of 66 children (74%) were alive and in care. Eighty-nine adults (15%) and 11 children (17%) were lost to follow-up. There was a statistically significant difference in the baseline CD4+ T-cell counts between patients who were lost to follow-up and those who were not; 86/mm3 versus 64/mm3, respectively (p=0.05). Other baseline characteristics were comparable between these two groups (data not shown). One hundred and thirteen adults (19%, Table 2) and six children (9%) died. Of the 113 adult deaths, 49 (43%) occurred within the first month after the initiation of HAART and 78 (69%) occurred within the first two months. Three of the six children passed away during the first month of HAART.
As most patients died at home, the actual cause of death was often unknown.
There was no statistically significant difference in survival between patients who received tuberculosis treatment and those who did not (p=0.7).
Viral load, CD4+ T-cell response, and BMI in adults
No blood samples were available after the baseline sample in 81 of the 609 (13%) adults: 49/81 died and 32/81 were lost to follow-up before a second sample could be drawn. For the remaining 528 patients, on-treatment blood samples were available as well. A plasma HIV-RNA level lower than 400 copies/ml in at least one of those blood samples was seen in 494 of these 528 (94%) patients, and a plasma HIV-RNA level lower than 50 copies/ml was registered in 439 of 528 patients (83%, Fig. 2).
Longitudinal follow-up of the patients who achieved viral suppression showed that plasma HIV-RNA levels remained lower than 400 copies/ml in 400 of 494 (81%) patients, and lower than 50 copies/ml in 316 of 439 patients (72%). Virological failure (plasma HIV-RNA >1,000 copies/ml) occurred in 51 patients (10%). No further data after the initial viral suppression were available for 43 (9%) patients (Fig. 3).
Virological data in adults and children after reaching a plasma HIV-RNA level ≤400 copies/ml. Continued to have viral suppression is defined as having a plasma HIV-RNA levelbelow 50 copies/ml during successive measurements. The probability of experiencing virological failure was significantly higher in children than in adults (p<0.025)
At one-year follow-up, the plasma HIV-RNA level was lower than 400 copies/ml in 336 patients (336/609 ITT=55%, 336/407 OT=83%) and lower than 50 copies/ml in 283 patients (283/609 ITT=46%, 283/407 OT=70%).
The mean increase in the CD4+ T-cell counts was 168/mm3 (95% CI: 156–179, p<0.001) at week 24 and 236/mm3 (95% CI: 220–252, p<0.001) at week 52, resulting in a median CD4+ T-cell count of 297/mm3 (IQR 195–421 /mm3). Sixty-one percent of adults had a good immunological response (CD4 T-cell count >200/mm3) at week 24 and 74% at week 52 (on-treatment analysis).
The mean BMI had increased by 2.4 kg/m2 (p<0.001) at week 24 and by 3.5 kg/m2 (p<0.001) at week 52, resulting in a median BMI of 23.4 kg/m2 at week 52.
Viral load, CD4+ T-cell response, and weight in children
Apart from the seven children who either died or were lost to follow-up before week six, a plasma HIV-RNA level lower than 400 copies/ml was registered at some time during therapy in all 59 children, and a level lower than 50 copies/ml was registered in 52/59 children (88%). Plasma HIV-RNA levels remained under 400 copies/ml during consecutive tests in 42/59 children (71%); 33/52 children (63%) continued to have plasma HIV-RNA levels lower than 50 copies/ml. Virological failure occurred in 12 children (20%). No data were available for the remaining five children after reaching viral suppression. The probability of virological failure was significantly higher in children than in adults (p=0.03, Fig. 3).
At one-year follow-up, the plasma HIV-RNA level was lower than 400 copies/ml in 35 children (35/66 ITT=53%, 35/49 OT=71%) and lower than 50 copies/ml in 30 children (30/66 ITT=45%, 30/49 OT=61%).
The mean CD4% increase was 12.6 (95% CI: 10.4–14.8, p<0.001) at week 24 and 17.6 (95% CI: 14.1–21.1, p<0.001) at week 52. The median CD4% at week 52 was 26.3 (IQR 20.0–33.0). Fifty-three children (80%) achieved a CD4% of over 15 during the 52 weeks of follow-up and 53% of these children attained this response within six weeks.
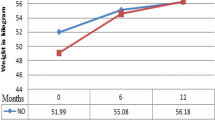
The children also gained weight during the study: the mean weight gain was 2.2 kg (95% CI: 1.8–2.6, p<0.001) at week 24 and 3.6 kg (95% CI: 3.2–4.1, p<0.001) at week 52.
Predictors of efficacy in adults
A Karnofsky score ≤50, a BMI in the lowest quartile (<17.1 kg/m2), and a CD4+ T-cell count <50/mm3 were all associated with death in the univariate analysis. In multivariate analysis, a Karnofsky score ≤50 at baseline (95% CI: 0.09–0.32, p<0.001) and a CD4+ T-cell count <50/mm3 (95% CI: 0.30–0.96, p= .04) remained as independent predictors of death.
When the CD4 T-cell count remained below 200/mm3 during the year of observation, it was classified as a poor immunological response. A Karnofsky score ≤50, a BMI in the lowest quartile (<17.1 kg/m2), a CD4+ T-cell count <50/mm3, and gender were associated with a poor immunological response in both the univariate and multivariate analyses.
A CD4+ T-cell count <50/mm3 at baseline was also associated with a statistically significant lower chance of attaining a plasma HIV-RNA level lower than 50 copies/ml (p=0.01).
Discussion
This study shows the feasibility of antiretroviral treatment administered and monitored by general practitioners in a primary healthcare setting in rural South Africa.
One year after the start of HAART, 89 of 609 adults (15%) were lost to follow-up and 113 adults had died (19%). Patients who did not show up for their scheduled visits were actively followed-up. It seemed that patients were often lost to follow-up due to labor migration, patient preference for traditional healers, or the lack of means to visit the clinic, but the exact numbers are unknown. There is no reason to believe that a considerable number of patients who were lost to follow-up had died, as their baseline characteristics were similar to the rest of the patients, apart from the baseline CD4+ T-cell count, which was even higher in the patients who were lost to follow-up.
Immunologic outcomes were good: the mean CD4+ T-cell count increase after one year was 236/mm3; previous studies showed increases in mean CD4+ T-cell counts ranging from 127/mm3 to 165/mm3 [5, 8–11].
In an on-treatment analysis at one-year follow-up, 83% of the adult patients had a plasma HIV-RNA level lower than 400 copies/ml and 70% of patients had a plasma HIV-RNA level lower than 50 copies/ml; ITT was 55% and 46%, respectively. These results compare well to the results of HAART in other settings. A historical meta-analysis of clinical trials on NNRTI-based triple combination therapy in antiretroviral-naïve patients in western settings showed a plasma HIV-RNA level lower than 50 copies/ml after 48 weeks of treatment in 51% of patients (ITT) [5]. An on-treatment analysis in a recent study on HAART in sub-Sahara Africa showed a plasma HIV-RNA level lower than 50 copies/ml in 61% of patients after 48 weeks of therapy (ITT 55%) [11]. Additionally, a meta-analysis of ten studies on the efficacy of antiretroviral therapy in resource-poor, urban settings showed similar virologic responses as that observed in our study [17]. However, with the current treatment options and low pill burden, outcomes in developed countries are clearly better today [18, 19].
A recent comparative study examining viral suppression in resource-poor and industrialized settings states that the virologic responses are similar in these two settings when correction is applied for the increased mortality rates in the resource-poor settings during the first months of therapy [20]. In our study, high mortality rates during the first months of HAART were also observed [17, 21]. Patients often sought help at an advanced stage of their disease; a lower BMI and CD4+ T-cell count in men suggests that men deferred coming to the clinic for even longer than women. As a low Karnofsky score and a low baseline CD4+ T-cell count were both independently associated with death, this partly explains the relatively low survival outcomes in the intention-to-treat analyses and points to a need for earlier intervention.
Most studies on the virologic and immunologic efficacy of HAART in sub-Saharan Africa were performed in urban areas [6–9, 17], whereas the patients included in our study lived in a rural environment with high unemployment rates and a high HIV prevalence. This distinction is important, as people living in remote areas often miss out on proper care and the process of scaling-up antiretroviral therapy in such settings in sub-Saharan Africa meets different challenges [10, 22]. A recent analysis showed that only 21% of the people in Mpumalanga estimated to be in need of HAART actually received it by the end of 2005 [23]. Apart from the difference between urban and rural settings, the socio-demographic data in our cohort (Table 1) are similar to those found in a previous study from Cape Town [24].
Virological failure occurred more often in children (20%) than in adults (10%). The reason for this finding is unclear and needs further evaluation. It is possible that the more complex dosing system using syrups instead of tablets has led to mistakes by caregivers, but other factors, such as specific adherence issues, may also have contributed to this trend. A recent report on fixed-dose combination antiretroviral therapy in children in resource-limited settings showed similar results to our study regarding the median CD4% gain [25]. The median follow-up time in that study was only 6 months however, and no longitudinal viral load testing was performed.
Independent predictors of death in adults were a low Karnofsky score and a low CD4+ T-cell count at baseline. In contrast to other studies [9, 10, 26, 27], we did not find a significant correlation between low body weight and death. All of the parameters included (low CD4+ T-cell count, Karnofsky score, BMI, and gender) were independently associated with a poor immunological response. The breakdown analysis of CD4+ T-cell counts at baseline also showed a clear trend towards higher survival rates with increasing CD4+ T-cell counts (Table 2), supporting a previous study which states that initiating HAART in patients with CD4+ T-cell counts of over 200 is cost-effective [28]. Another report found a low baseline CD4+ T-cell count to be predictive of a poor, virological outcome [11]; this was confirmed by our data.
Unlike many other studies on HAART programs in Africa [8–11], we measured the viral load in individual patients throughout the treatment period. In contrast to the opinion of some authors [29, 30], our data indicate that longitudinal viral load testing is possible in developing countries. In our opinion, viral load testing plays a significant role in clinical decision-making during HAART. The lack of routine viral load testing can lead to the misdiagnosis of treatment failure and may result in unnecessary regimen changes [31, 32]. An unexpected rise in plasma HIV-RNA levels can indicate poor patient adherence to treatment or viral resistance, and can be used as a criterion for extra counseling or the changing of treatment regimens.
The HAART program at the Ndlovu Medical Centre provides extensive counseling before and during treatment. Since most of the staff and all counselors are local people, some HIV-positive themselves, they are able to comprehend and discuss many problems that patients in the program encounter. Given that adherence is vital to good treatment outcomes [10, 33–35], we feel that the emphasis on counseling and adherence were essential for the program’s success. However, even in this closely monitored, strictly counseled, and fully funded environment, with no monetary costs for the patient, over 15% of patients were lost to follow-up. Many domestic, social, and educational hurdles still need to be overcome in order to achieve a more favorable compliance rate.
We recognize that this study was limited by the relatively short follow-up period.
In summary, this study shows that the treatment and monitoring of HIV-infected patients by general practitioners in rural areas in sub-Saharan Africa can result in good virological, immunological, and clinical outcomes. In order to achieve even more favorable results, early mortality should be reduced and efforts should be made to start HAART at an earlier stage of disease. These data support the viability of and the need for large-scale initiatives to address the enormous threat that AIDS has become worldwide. Many questions remain unanswered, however, and future research should focus on the long-term outcomes of HAART and the possibilities for second and consecutive lines of treatment in an attempt to predict and control viral resistance.
References
World Health Organization (WHO) (2004) Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. WHO, Geneva, Switzerland. Available online at: http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf
Panel on clinical practices for the treatment of HIV infection, convened by the DHHS (2008) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, January 29, 2008. Available online at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
Panel on clinical practices for the treatment of HIV infection, convened by the DHHS (2008) Guidelines for the use of antiretroviral agents in pediatric HIV infection, February 28, 2008. Available online at: http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf
Egger M, May M, Chêne G, Philips AN, Ledergerber B, Dabis F, Costagliola D, D’Arminio Monforte A, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA; ART Cohort Collaboration (2002) Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 360:119–129
Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F (2001) Overview of the effectiveness of triple combination therapy in antiretroviral-naïve HIV-1 infected adults. AIDS 15:1369–1377
Laurent C, Diakhaté N, Gueye NFN, Touré MA, Sow PS, Faye MA, Gueye M, Lanièce I, Touré Kane C, Liégeois F, Vergne L, Mboup S, Badiane S, Ndoye I, Delaporte E (2002) The Senegalese government’s highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS 16:1363–1370
Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, Reuter H, Ntwana N, Goemaere E (2004) Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS 18:887–895
Wools-Kaloustian K, Kimaiyo S, Diero L, Siika A, Sidle J, Yiannoutsos CT, Musick B, Einterz R, Fife KH, Tierney WM (2006) Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS 20:41–48
Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, George E, Kenel-Pierre S, Wright PF, Gulick R, Johnson WD Jr, Pape JW, Fitzgerald DW (2005) Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med 353:2325–2334
Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, Karungi G, Szumilin E, Balandine S, Fedida G, Carrieri MP, Spire B, Ford N, Tassie JM, Guerin PJ, Brasher C (2006) Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet 367:1335–1342
DART Virology Group and Trial Team (2006) Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS 20:1391–1399
World Health Organization (WHO) (2006) Progress on global access to HIV antiretroviral therapy. A report on “3by5” and beyond, March, 2006. Available online at: http://www.who.int/hiv/fullreport_en_highres.pdf
Hosegood V, Vanneste AM, Timaeus IM (2004) Levels and causes of adult mortality in rural South Africa: the impact of AIDS. AIDS 18:663–671
Human Sciences Research Council of South Africa (2005) The South African national HIV prevalence, HIV incidence, behaviour and communication survey, 2005. Available online at: http://www.hsrc.ac.za/media/2005/11/20051130_1Factsheet2.html
Department of Health, Republic of South Africa (2004) National HIV and syphilis antenatal sero-prevalence survey in South Africa, 2004. Available online at: http://www.doh.gov.za/docs/reports/2004/hiv-syphilis02.pdf
National Department of Health, South Africa (2004) National antiretroviral treatment guideline, 2004. Available online at: http://hivinsite.ucsf.edu/doc/cr09-sf-01.doc
Ivers LC, Kendrick D, Doucette K (2005) Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis 41:217–224
Gulick RM, Ribaudo HJ, Shikuma CM, Lalama C, Schackman BR, Meyer WA 3rd, Acosta EP, Schouten J, Squires KE, Pilcher CD, Murphy RL, Koletar SL, Carlson M, Reichman RC, Bastow B, Klingman KL, Kuritzkes DR; AIDS Clinical Trials Group (ACTG) A5095 Study Team (2006) Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 296:769–781
Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK (2006) Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 354:251–260
ART-LINC Collaboration and ART-CC groups (2006) Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet 367:817–824
van der Sande MA, Schim van Der Loeff MF, Aveika AA, Sabally S, Togun T, Sarge-Njie R, Alabi AS, Jaye A, Corrah T, Whittle HC (2004) Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr 37:1288–1294
Van Damme W, Kober K, Laga M (2006) The real challenges for scaling up ART in sub-Saharan Africa. AIDS 20:653–656
Nattrass N (2006) South Africa’s “rollout” of highly active antiretroviral therapy: a critical assessment. J Acquir Immune Defic Syndr 43:618–623
Coetzee C, Nattrass N (2004) Living on AIDS treatment: a socio-economic profile of Africans receiving antiretroviral therapy in Khayelitsha, Cape Town. CSSR Working paper no. 04/071. Available online at: http://www.cssr.uct.ac.za/pubs_cssr.asp
O’Brien DP, Sauvageot D, Zachariah R, Humblet P; Medecins Sans Frontieres (2006) In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS 20:1955–1960
Chan KC, Yip B, Hogg RS, Montaner JS, O’Shaughnessy MV (2002) Survival rates after the initiation of antiretroviral therapy stratified by CD4 cell counts in two cohorts in Canada and the United States. AIDS 16:1693–1695
Erikstrup C, Kallestrup P, Zinyama R, Gomo E, Mudenge B, Gerstoft J, Ullum H (2007) Predictors of mortality in a cohort of HIV-1-infected adults in rural Africa. J Acquir Immune Defic Syndr 44(4):478–483
Badri M, Cleary S, Maartens G, Pitt J, Bekker LG, Orrell C, Wood R (2006) When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther 11:63–72
Koenig SP, Kuritzkes DR, Hirsch MS, Léandre F, Mukherjee JS, Farmer PE, del Rio C (2006) Monitoring HIV treatment in developing countries. BMJ 332:602–604
Colebunders R, Moses KR, Laurence J, Shihab HM, Semitala F, Lutwama F, Bakeera-Kitaka S, Lynen L, Spacek L, Reynolds SJ, Quinn TC, Viner B, Mayanja-Kizza H (2006) A new model to monitor the virological efficacy of antiretroviral treatment in resource-poor countries. Lancet Infect Dis 6:53–59
Basenero A, Castelnuovo B, Birabwa E, John L, MacAdam K, Schlech W, Kambugu A (2007) Inadequacy of clinical and immunological criteria in identifying virologic failure of 1st line ART: the Ugandan experience. In: Program and abstracts of the 4th International Aids Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia July, 2007, abstract WEAB102
Hosseinipour M, van Oosterhout J, Weigel R, Mzigangira D, Saukila N, Mhango B, Phiri R, Phiri S, Kumwenda J (2007) Validating clinical and immunological definitions of antiretroviral treatment failure in Malawi. In: Program and abstracts of the 4th International Aids Society (IAS) Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia, July 2007, abstract WEAB101
Mannheimer S, Friedland G, Matts J, Child C, Chesney M (2002) The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 34:1115–1121
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR (2006) Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA 296:679–690
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Barth, R.E., van der Meer, J.T.M., Hoepelman, A.I.M. et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis 27, 977–984 (2008). https://doi.org/10.1007/s10096-008-0534-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0534-2