Abstract
Fluorescence polarization assay (FPA) is a method that has been used for the diagnosis of brucellosis in animals for many years. To test its possible usefulness for the diagnosis of human brucellosis, 230 sera from patients with clinical signs of brucellosis and positive serological tests (Rose Bengal, Standard Agglutination Test, iELISA), and 305 sera from a healthy population with no clinical/epidemiological/serological evidence were examined with FPA. By using ROC analysis, the cut-off value was estimated at 99 mP, with 93.5% sensitivity (95% CI 89.5–96.3) and 96.1% specificity (95% CI 93.2–97.9). The pairwise comparison of ROC curves between FPA and iELISA and between FPA and RBT revealed no significant statistic difference (P < 0.05). On the contrary it revealed a significant statistic difference between FPA and SAT (P > 0.05). SAT also had the lowest sensitivity (81.7%) among the three tests used in case definition while iELISA had a sensitivity of 90.8% and RBT a sensitivity of 88.7%. The Kappa analysis showed that FPA has a very good agreement (0.92) with the “status of the disease” and with iELISA (0.837). According to our results, FPA seems to be a valuable method for the diagnosis of brucellosis in humans. Taking into consideration the advantages of the method such as the speed of results obtaining, the objectivity of results interpretation, as well as the cost, FPA could be considered as a replacement for other established methods. However, further studies are needed to assess the reproducibility of FPA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brucellosis is an important zoonosis in many parts of the world. The Mediterranean basin, the Arabian Peninsula, the Indian subcontinent, Central (Mexico) and South America, are the areas with the highest reported incidence of brucellosis in humans [1].
The epidemiology of brucellosis differs between endemic and not endemic areas. In endemic areas, the disease is transmitted mostly by ingestion of contaminated diary products (unpasteurized milk and its products: white cheese, fresh butter or cream, etc.), direct contact with infected animals and their discharges and poor personal hygiene. On the contrary, in non-endemic areas, the infection is almost exclusively occupational and veterinarians, abattoir workers, farmers and laboratory personnel are in the highest risk category. The usual ways of infection is through skin, conjunctiva and respiratory system [2]. There are also a few reports of human-to-human transmission by tissue or bone-marrow transplantation, blood transfusion and sexual contact [3], but these types of infection are very rare and not important. The clinical diagnosis of human brucellosis may be very difficult due to variable clinical symptoms, chronicity of the disease, occurrence of subclinical, atypical or localized infections, paucity of the characteristic symptoms, previous antibiotic therapy and Brucella species. Thus, bacteriological and serological examinations are usually essential for confirmation of diagnosis [4, 5].
The diagnosis of brucellosis is made with certainty when Brucella spp. is isolated from blood, bone-marrow or tissue cultivation. Since the primary isolation is difficult, time-consuming, requires special media and a Biosafety Level III cabin, and presents a risk for laboratory personnel, the confirmation of diagnosis in humans is based mainly on serology.
The standard tube agglutination test (SAT) still remains the basis from which all other methods are compared. The limitations of SAT are that it cannot be used for large numbers of sera (especially in small general hospitals covering large endemic rural areas as happens in Greece) and it is both a work and time intensive test. Additionally, it has low sensitivity (Sn) in chronic and localized cases and it often gives false positive results because of cross-reaction with other gram-negative bacteria [6, 7].
The Rose Bengal test (RBT), which is another agglutination test, has high sensitivity and relatively low specificity (Sp) [8]. It is quick, inexpensive and easily performed and can be used as a rapid screening test in endemic areas [9]. The RBT also gives false positive reactions with other gram negative bacteria, albeit to a lesser extent than SAT [10].
The Brucella enzyme linked immunosorbent assay (ELISA) has been reported to be highly sensitive and specific and its major advantage is the determination of specific IgG, IgM and IgA brucella antibodies in blood, serum and CSF [11]. Moreover, ELISA has a higher sensitivity and detects different classes of immunoglobulins than SAT [12]. The problem of cross-reaction with other bacteria is even more less in ELISA. Most ELISAs, including our commercial ELISA kit, use internal cell protein antigens (cytoplasmic antigens) and the anti-human IgG factor in order to avoid the problem of cross-reactions with other bacteria [4, 13].
Fluorescence polarization assay (FPA) was validated for the diagnosis of bovine [14], ovine [15] and swine [16] brucellosis. It measures the antibody binding to antigen directly, based on a fluorescent dye attached to a small antigen or an antibody fragment, which is excited by plane-polarized light in a specific wavelength. When the molecular size of the antigen remains unchanged (absence of antibody), the rate of rotation and consequently the light polarization also remains constant. On the other hand, when the molecular size is increased (existence of an antigen-antibody complex), its rate of rotation is reduced and the light polarization becomes high. The change of this rate can be measured by a fluorescence polarization analyser and the result is expressed in millipolarization (mP) units.
The aim of the present study is the comparison of FPA performance with that of RB, SAT and ELISA (IgM and IgG) and its possible application in the diagnosis of human brucellosis in a region where the infection is endemic both in animals and humans.
Materials and methods
Samples
Three hundred and seventeen blood samples were collected from patients who attended the local hospitals in the region of Thessaly during the years 2002–2004 with clinical features suggesting brucellosis and had been referred to the laboratory with possible brucellosis: signs and symptoms with at least one risk factor (occupation, animal contact, consumption of unpasteurized milk, etc.). The confirmation of diagnosis was made by laboratory examination. All samples were examined by SAT, Rose Bengal and ELISA (IgG and IgM) tests.
Three hundred and eighteen blood samples were also collected from healthy individuals who visited the same hospitals in the same period as blood donors. All these samples were also examined with SAT, Rose Bengal and ELISA (IgG and IgM) tests.
Definitions
Brucellosis positive (BP)
Patients who exhibited (1) clinical signs compatible with brucellosis, (2) at least one risk factor and (3) a positive reaction at least in two of the current used serological tests, were characterized as brucellosis positive.
Brucellosis negative (BN)
Healthy individuals who had (1) no clinical signs suggesting brucellosis, (2) no epidemiological link and (3) negative reaction to all the serological tests were characterized as brucellosis negative. Fluorescence polarization assay was then performed in all brucellosis positive and brucellosis negative blood samples.
Serological tests
All blood samples were centrifuged at 1,000 g for 20 min and serum was separated from the clot, aliquoted and stored at −20°C until tested.
Standard tube agglutination test
The SAT test was performed by the tube dilution method as described by Alton et al. [17] with commercial Brucella abortus antigen (Cypress Diagnostics CV, Langdorp, Belgium). All samples were diluted from 1:20 to 1:10,240 and incubated with antigen for 24 h at 37°C. The agglutination reactions were read by indirect light against a white background and the results were scored as follows: 0 = no agglutination (negative), 1+ = 25% agglutination, 2+ = 50% agglutination, 3+ = 75% agglutination and 4+ = 100% agglutination. The titer of the sample was suggested to be the highest dilution in which there was 50% agglutination (2+). All samples that had a titer of ≥1:160, were suggested as positive.
Rose Bengal Test
The RB test was performed as described by Alton et al. [17] with commercial B. abortus antigen (Synbiotics Corporation, Europe SAS, Lyon, France). 30 μl of serum was mixed in room temperature with 30 μl of antigen on a flat glass plate divided into 40 square (15 mm) cells and shook for 4 min on a single-directional to and fro rocker (30 oscillations per minute). Results were considered to be positive in the presence and negative in the absence of agglutination.
ELISA (IgG and IgM) test
Both IgG and IgM ELISA tests were performed using commercial kits (Panbio Ltd, Windsor, Qld, Australia) [18]. The ELISA microplate testing was performed exactly as described in the manufacturer’s procedure manual. All the steps of the ELISA procedure (washing, incubation and absorption reading) and the final estimation of the titer as positive or negative were done automatically using an automated EIA analyser (Triturus Grifols SA, Barcelona, Spain) and the Triturus software (Triturus Ver 3.00b), respectively.
Fluorescence polarization assay
FPA was conducted in 96-well flat bottom black polysterine microtiter plates, type COS96fb manufactured by Corning USA. Initially, 200 μl of dilution buffer was placed in each of the three wells in the first column (A1, B1, and C1) and were designated buffer controls. In each of the remaining wells, 180 μl of dilution buffer and 20 μl of test sera were added.
The dilution buffer was provided by the manufacturer in 25X concentrate form and the working dilution was prepared using ultra-clean sterile water. In each microplate, positive and negative control sera of bovine origin, which had been provided by the manufacturer, were used in duplicate. Buffer and serum samples were mixed, setting the microplate on a rotating microplate shaker for 2 min at room temperature. After the initial mixing, a background reading was taken at fluorescence polarization mode by a multi-mode microplate reader (Tecan Genios Pro) connected to a laptop computer. Subsequently, 10 μl of antigen (O-polysaccharide from B. abortus strain 1119.3 prepared and conjugated with FITC) as described by Lin and Nielsen was added to all wells [19]. After mixing for 3 min at room temperature, a second reading was taken. The reader automatically subtracted the background reading and calculated a value for every sample in millipolarization units (mP). The reagents used in this assay were manufactured by Diachemix, Whitefish Bay, WT, USA and supplied by Prionics AG Switzerland.
The fluorescence polarization reader was calibrated by the use of blank and low polarized standards, which were included in the kit together with the antigen. The calibration was made automatically; the instrument takes the value of the low polarized standard (fixed at 25 mP) and calculates an internal compensation factor (G-factor) which is utilized in the equation for calculating the final results. The measurement parameters of the instrument were set for gain at 55, integration time at 40 μs and at 10 flashes per well per second. The filter for excitation wavelength was at 485 nm and 535 nm for emission. The results of each microplate measurement were accepted if the mP values of positive control were >180 mP, of negative control 60–85 mP and buffer control >70 and <80 mP according to the manufacturer’s instructions.
Statistical analysis
All the data were entered in a database created in the statistical program MedCalc (version 8.0.1.0). The cut-off value, the Se, the Sp and the area under curve (AUC) and their 95% CI were determined with ROC analysis using the statistical program MedCalc. The ROC curves comparison, the pairwise comparison of them and the k-value were estimated by using the MedCalc software.
Results
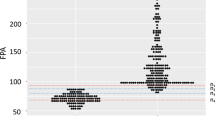
From the 317 patients with possible brucellosis, 230 gave positive results at least in two of the tests performed (RBT, SAT, ELISA IgG and IgM) and were considered as brucellosis positive (BP). From the 318 healthy individuals with no brucella evidence, 305 gave negative results in all same tests and were considered as brucellosis negative (BN). The 230 BP and the 305 BN sera results were used for the ROC analysis. As shown in Fig. 1, the area under curve for the FPA was estimated at 98.1 (95% confidence interval 96.6–99.1 and standard error 0.006).
A cut-off value of 99 mP was selected because it gave the maximum sum of sensitivity and specificity (189.6) and was considered to be the optimum value. Any serum showing a value of exactly 99 mP was considered as negative.
For this cut-off value the FPA detected 215 from the 230 BP blood sera with values ranging from 100 to 310 mP and gave 15 false-negative results ranging from 87 to 99 mP, while from the 305 negative blood sera detected 293 samples with values ranging from 35 to 99 mP and gave 12 false-positive results ranging from 100 to 111 mP (Table 1). One sample gave a result of 35 mP, which was the same in repeated measurements. The specific reasons of having such a low mP value still remain unclear. Perhaps there were some unknown factors in the serum which inhibited the fluorescence of the antigen.
For the same cut-off value, the sensitivity and the specificity were determined to be 93.5% (95% confidence interval 89.5–96.3) and 96.1% (95% confidence interval 93.2–97.9) respectively. For this value, the positive likelihood ratio was also 23.76 and the negative likelihood ratio was 0.07. The sensitivity, specificity and AUC of ROC analysis of all the tests performed are presented in Table 2.
The comparison of ROC curves of all the tests performed is presented in Fig. 2, while the results of pairwise comparison of ROC curves are presented in Table 3.
The pairwise comparison of ROC curves, in particular the comparison of FPA vs. ELISA, FPA vs. RBT and ELISA vs. RBT, did not reveal any significant statistic difference (P > 0,05), as compared to FPA vs. SAT and ELISA vs. SAT, which revealed significant statistic difference (P < 0,05). Finally the comparison of RBT vs. SAT remained unsolved (P = 0,05).
Kappa statistic was used to assess the agreement of the results of each test with the BP patients. The agreement of the results of every test with all the others was also assessed. According the interpretation of Altman [20] the strength of agreement is very good when the K value is 0.81–1, good at 0.61–0.8, moderate at 0.41–0.6, fair at 0.21–0.4 and poor when the K value is 0–0.2. The results of Kappa statistic revealed a very good agreement for FPA vs. BP, FPA vs. ELISA, FPA vs. RBT, ELISA vs. BP, ELISA vs. RBT, SAT vs. BP and RBT vs. BP. They also revealed a good agreement for SAT vs. FPA, SAT vs. ELISA andd SAT vs. RBT. The results of Kappa statistic are presented in Table 4. The results of Kappa analysis are presented in Table 4.
Discussion
The clinical signs and symptoms of brucellosis are usually severe but may be confused with various other diseases that require a different therapeutic approach. Therefore an easily performed, cheap and reliable test would be of great value in differentiating these disorders. A considerable number of serological tests have been developed since 1897, when Wright and Smith described the first test for diagnosis of brucellosis. Many of these tests were modified in various ways in order to increase the performance.
The most economical and most widely used laboratory tests in diagnosis of the disease are the agglutination tests SAT and RBT. Among these tests, SAT still remains the reference test for the diagnosis of human brucellosis in many countries of the world. Studies comparing the results of SAT and RBT have reported a considerable concordance [21] and the interpretation of their results is largely subjective. Many other serological tests have been widely studied such as the Coomb’s test, the 2-mercaptoethanol test, the complement fixation tests (warm and cold), the primary binding assays (indirect and competitive ELISA), the radio-immunoassay (RIA), the passive hemagglutination test and the Brucella fluorescent antibody test. The advantages of these tests are the high sensitivity and specificity and the ability to detect the non-agglutinating antibodies and their immunoglobulin classes which is of great importance in the chronic and localized forms of the disease but some of these are complex, laborious and expensive and may involve radiation hazards [12]. Among the tests, ELISA is the most sensitive and specific [22].
The isolation of Brucella spp. by blood, tissue or bone-marrow cultures is the only mean of definitively proven infection while a single serological test is insufficient for diagnosis. At least two of the later have to be used in parallel or be combined to avoid false negative or false positive results, respectively [17].
In addition to serology, molecular means such as polymerase chain reaction and a great number of modified PCR techniques (PCR-RFLP, AP-PCR, REP-PCR, ERIC-PCR, RAPD-PCR and real-time PCR) are used in many laboratories for the diagnosis of human brucellosis [22]. These means allow the detection of specific brucella DNA sequences by amplification of very few molecules of DNA. The disadvantages of these techniques are that they are expensive, time consuming and require specialized laboratories and personnel. Thus they cannot be used in many regions of the world, except developed countries.
Despite the great number of laboratory methods, conclusive evidence of infection is still only possible by culture. However, serology, because of its advantages such as lower cost, the rapid obtaining of objective results and its ability to be performed by nonspecialized personnel, still has an important role to play in diagnosis of the disease.
FPA is a widely used diagnostic method in animal brucellosis and has many advantages: e.g., it is a rapid method with only one-step dilution of serum, does not depend on the kind of sample (whole blood, serum or plasma), does not require specialized personnel, is relatively inexpensive and, because data are obtained electronically, it is an objective test. [23, 24]. Another advantage of the method is that it gives the ability to be used with appropriate modifications for a particular area or epidemiological characteristics, by increasing or decreasing the cut-off value.
We intended to test FPA in Greece, because it is a new serological method that has not been used in diagnosis of human brucellosis widely and because this infection is endemic in our country [25, 26]. For these reasons, we selected to confirm the cut-off value of the assay by using positive and negative sera as described previously and comparing it with SAT, RBT and ELISA.
With the cut-off value of 99 mP, the specificity was 96.1% and the sensitivity was 93.5%. Although the specificity of FPA defined in this study was the lowest compared with that of other tests performed, it must be considered to be high especially in an endemic country like Greece where the officially reported cases are 21/1,000,000 per year [27], but the true incidence of brucellosis is 21–30/100,000 [28]. Since the selection criterion for negative reference sera was the results of the other tests used in the study, it is expected that their specificity would be at 100% and this perhaps would tend to bias both the sensitivity and specificity results in favour of the tests used in definition of BN samples. The defined sensitivity and specificity of FPA in this study compared with that of the other tests defined in previous studies appears to be equal or higher than them. In a review of the literature, Al Dahouk et al. presents the sensitivity and specificity of the other tests referred in previous studies as following: iElisa: Se 94–100% and Sp 97–100%, RBT: Se 90–95% and Sp 84–98% and SAT: Se 51–96% and Sp ≤100%, depending on the presence of IgM titers with nonspecific activity [22]. The 15 false-negative results might be related to bad affinity of the antigen with the antibody or with low titer of serum antibodies as happens in all the serological tests that have a sensitivity <100%. The 12 false-positive sera might be correlating to serum quality (small clots or other particles present in the serum could impair the passage of light beams and interfere with the measurements). Another reason could be the cross-reactions with antibodies produced in response to bacteria with structurally related antigens, possibly including Yersinia enterocolitica O:9, Escherichia coli O:157, Salmonella serotypes of Kaufmann-White group N and Stenotrophomonas multopfilia [5]. It must be pointed out that in Greece, the cases of Y. enterocolitica infection are very few and are related mainly with children suffering from b-thalassaemia. The infection of E. coli O:157 is also very rare with only one case reported in 2006. Thus the probability of false-positive results related to cross-reacting bacteria is very limited. For the same cut-off value, the sensitivity of FPA was 93.5%, which is also deemed a high percentage.
The comparison of our results and Lucero et al. [18], revealed small differences in sensitivity and specificity values (Se: 93.5 vs. 96.1 and Sp: 96.1 vs. 97.9) and a remarkable difference between the cut-off values of the two studies (99 vs. 72). The difference in the sensitivity values between our study and Lucero’s could be due to the population tested. In the Lucero study, the positive population is a culture-proven population and a population where all serology had to be positive, while in our study, only serology was used with two tests needing to be positive.. The difference in the cut-off values could be due to the local conditions or the different process of the method between the two studies. Lucero et al. used a transparent glass tube with 1 ml buffer, while we used only 100 μl of buffer in each black opaque well of a 96-well microplate. The reading instruments were also different as different was the calibration procedure. Finally, the different buffer we used could be responsible for this large discrepancy for the FPA cut-off between both tests. Lucero et al. [18] used a single tube device, while we performed the test using a 96-well FPA reader. The use of such a reader makes the FPA more rapid, easier and cheaper than the use of a single tube one.
The FPA and iELISA were the tests with the higher sensitivity. Comparing the sensitivities of the current used tests, FPA had the higher value, while SAT had the worst. ELISA had a slightly lower Se than that referred to in bibliography, while SAT’s and RBT’s Se were according with it [1, 29]. For the sum of Se+Sp, the results were in the following order of magnitude: iELISA > FPA > RBT > SAT.
The AUC values of all the tests (all greater than 90%) show that these tests have high discriminatory ability since their AUC value is between 90 and 100% [30]. The highest AUC value of FPA indicates that in 98.1% of the cases, the test will correctly identify a patient as brucellosis positive if his serum has a value greater than the determined cut-off.
The pairwise comparison of the ROC curves revealed that FPA is in agreement uppermost with iELISA and secondarily with RBT (no significant statistic difference) and is significantly different from SAT. The results obtained by FPA with Kappa analysis were in a very good agreement (0.92) both with the “status of the disease” as defined previously or with that obtained by iELISA (0.919) and RBT (0.826) and was in good agreement with those obtained by SAT (0.77).
The results of this study revealed that FPA has an accuracy equal to iELISA’s and RBT’s and is a significantly superior diagnostic test to the SAT; the latter had the worst accuracy of all the current used tests. The fact that the results of FPA are equivalent to those from iELISA is in accordance with other studies comparing FPA with standardized tests used in the diagnosis of animal brucellosis [24, 29].
According to our results, FPA seems to be of significant value for the diagnosis of human brucellosis, even though there is not much experience with the laboratory use of the assay. Since there are many differences between animal and human disease (localized forms, multi-system complications, etc.) and the nutrition of each host, further studies of FPA are needed. Such studies should be performed in various different settings such as endemic and not endemic areas, acute, chronic, or localized form of the disease, and bad nutritional habits or pathological situations (high levels of cholesterol, triglycerides, etc.) that could have an effect to the estimation of the polarization. It is also necessary that the reproducibility of the test be evaluated with further studies in order for FPA to be introduced as a routine test. Moreover, it would be interesting to test patients with Y. enterocolitica O:9 and Salmonella group N infections with FPA to document the existence of cross-reaction in a future study. It would also be very interesting to see what the specificity of FPA is in patients showing clinical signs, but without brucellosis and without pre-screening of samples with serology; although, due to lack of sensitivity with bacterial culture or PCR, it is difficult to demonstrate with certainly that such people are free of disease.
Abbreviations
- RBT:
-
Rose Bengal Test
- SAT:
-
Serum agglutination test
- FPA:
-
Fluorescence polarization assay
- BP:
-
Brucella positive
- BN:
-
Brucella negative
References
Young JE (1995) An overview of human brucellosis. Clin Infect Dis 21:283–290
Araj GF (1999) Human brucellosis: a classical infectious disease with persistent diagnostic challenges. Clin Lab Sci 12(4):207–212
Corbel MJ (1997) Brucellosis: an overview. Emerg Infect Dis 3(2):213–221
Hurtado R (2000–2001) Brucellosis: new and old issues regarding diagnosis and management, Harvard Medical School, Boston, MA, USA. http://www.mgh.harvard.edu/id/images/brucellosis.pdf. Cited 31 January 2001
Corbel MJ, MacMillan AP (1998) Brucellosis. In: Hausler N Jr, Sussman M (eds) Topley and Wilson’s microbiology and microbial infections (bacterial infections). Arnold, London, pp 819–847
Young EJ (1991) Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis 13:359–372
Moyer NP, Evins GM, Pigott NE, Hudson JD, Farshy CE, Feely JC, Hausler WJ Jr (1987) Comparison of serologic screening tests for Brucellosis. J Clin Microbiol 25(10):1969–1972
Ruiz-Mesa JD, Sanchez-Gonzalez J, Reguera JM, Martin L, Lopez-Palmero S, Colmenero JD (2005) Rose Bengal test: diagnostic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clin Microbiol Infect 11:221–225
Dabdoob WA, Abdulla ZA (2000) A panel of eight tests in the serodiagnosis and immunological evaluation of acute brucellosis. East Mediterr Health J 6(2/3):304–312
WHO (2003) Brucellosis in humans and animals. WHO Guidance 2003, WHO, Washington, DC, 30 pp
Araj GF, Lulu AR, Khateeb ML, Saadah MA, Shakir RA (1988) ELISA versus routine tests in the diagnosis of patients with systemic and neurobrucellosis. APMIS 96:171–176
Memish ZA, Almuneef M, Mah MW, Qassem LA, Osoba AO (2002) Comparison of the brucella standard agglutination test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn Microbiol Infect Dis 44:129–132
Pappas G, Akritidis A, Bosilkovski M, Tsianos E (2005) Brucellosis. N Engl J Med 352(22):2325–2336
Nielsen K, Gall D, Lin M, Massangill C, Samartino L, Perez B, Coats M, Hennager S, Dajer A, Nicoletti P, Thomas F (1998) Diagnosis of bovine brucellosis using a homogenous fluorescence polarization assay. Vet Immunol Immunopathol 66:321–329
Nielsen K, Gall D, Smith P, Balsevicious S, Thomas F, Tan S, Garrido F, Duran-Duran M, Biancifiori F, Davis D, Elzer P, Kenny K, Heneghan T, Simard C, Dajer A, Luna E, Samartino L (1999) Evaluation of the FPA for detection of ovine and caprine antibody to B. melitensis, antibody to B. abortus in bison and application of the FPA to detection of bovine antibody in EDTA treated blood from a B. abortus infected herd. Canadian fluorescence polarization report, Animal Diseases Research Institute, Canadian Food Inspection Agency, Ottawa, ON, Canada, pp 1–6
Nielsen K, Gall D, Smith P, Vigliocco A, Perez B, Samartino L, Nicoletti P, Dajer A, Elzer P, Enright F (1999) Validation of the fluorescence polarization assay as a serological test for the presumptive diagnosis of porcine brucellosis. Vet Microbiol 68:245–253
Alton GG, Jones LM, Angus RD, Verger JM (1988) Techniques for the Brucella Laboratory. Institut National de la Recherche Agronomique, Paris
Araj GF, Kattar MM, Fattouh LG, Bajakian KO, Kobeissi SA (2005) Evaluation of the PANBIO Brucella Immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays for diagnosis of human brucellosis. Clin Diagn Lab Immunol 12(11):1334–1335
Lin M, Nielsen K (1997) Binding of Brucella abortus lipopolysaccharide O-chain fragment to a monoclonal antibody. Biol Chem 272:2821–2827
Altman DG (1995) Practical statistics for medical research. Chapman and Hall, London, 404 pp
Mert Ozaras AR, Tabak F, Bilir M, Yilmaz M, Kurt C, Ongoren S, Tanriverdi M, Ozturk R (2003) The sensitivity and specificity of Brucella agglutination tests. Diagn Microbiol Infect 46:241–243
Al Dahouk S, Tomaso H, Nockler K, Neubauer H, Frangoulidis D (2003) Laboratory-based diagnosis of brucellosis: a review of the literature. Part II: serological tests for Brucellosis. Clin Lab 49:577–589
Lucero NE, Escobar GI, Ayala SM, Paulo PS, Nielsen K (2003) Fluorescence polarization assay for diagnosis of human brucellosis. J Med Microbiol 52:883–887
McGiven JA, Tucker JD, Perrett LL, Stack JA, Brew SD, McMillan AP (2003) Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT and iELISA. J Immunol Methods 278:171–178
Hadjichristodoulou Ch, Soteriades E, Goutzianna G, Loukaidou M, Babalis Th, Antoniou M, Delagramaticas J, Tselentis Y (1999) Surveillance of brucellosis in a rural area of Greece: application of the Computerised Mapping Programme. Eur J Epidemiol 15:277–283
Hadjichristodoulou Ch, Papatheodorou Ch, Soteriades E, Panagakos G, Kastritis I, Goutzianna G, Charvalos E, Tselentis Y (1999) Epidemiological study of brucellosis in eight Greek villages using a Computerised Mapping Programme. Eur J Epidemiol 15:671–680
Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infect Dis 6:91–99
Taleski V, Zerva L, Kantardjiev T, Cvetnic Z, Erski-Biljic M, Nikolovski B, Bosnjakovski J, Katalinic-Jankovic V, Panteliadou A, Stojkoski S, Kirandziski T (2002) An overview of the epidemiology and epizootiology of brucellosis in selected countries of central and southeast Europe. Vet Microbiol 90:147–155
Minas A, Stournara A, Minas M, Papaioannou A, Krikelis V, Tselepidis S (2005) Validation of fluorescence polarization assay (FPA) and comparison with other tests used for diagnosis of B. melitensis infection in sheep. Vet Microbiol 111:211–221
Greiner M, Pfeiffer D, Smith RD (2000) Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45:23–41
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konstantinidis, A., Minas, A., Pournaras, S. et al. Evaluation and comparison of fluorescence polarization assay with three of the currently used serological tests in diagnosis of human brucellosis. Eur J Clin Microbiol Infect Dis 26, 715–721 (2007). https://doi.org/10.1007/s10096-007-0363-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0363-8