Abstract
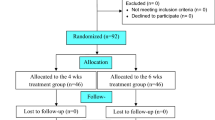
Despite rather strict recommendations for antibiotic treatment of disseminated Lyme borreliosis (LB), evidence-based studies on the duration of antibiotic treatment are scarce. The aim of this multicenter study was to determine whether initial treatment with intravenous ceftriaxone (CRO) for 3 weeks should be extended with a period of adjunct oral antibiotic therapy. A total of 152 consecutive patients with LB were randomized in a double-blind fashion to receive either amoxicillin (AMOX) 1 g or placebo (PBO) twice daily for 100 days. Both groups received an initial treatment of intravenous CRO 2 g daily for 3 weeks, followed by the randomized drug or PBO. The outcome was evaluated using the visual analogue scale at the follow-up visits. The final analysis included 145 patients, of whom 73 received AMOX and 72 PBO. Diagnoses of LB were categorized as either definite or possible, on the basis of symptoms, signs, and laboratory results. The diagnosis was definite in 52 of the 73 (71.2%) AMOX-treated patients and in 54 of the 72 (75%) PBO patients. Of the patients with definite diagnoses, 62 had neuroborreliosis, 45 arthritis or other musculoskeletal manifestations, and 4 other manifestations of LB. As judged by the visual analogue scale and patient records, the outcome after a 1-year follow-up period was excellent or good in 114 (78.6%) patients, controversial in 14 (9.7%) patients, and poor in 17 (11.7%) patients. In patients with definite LB, the outcome was excellent or good in 49 (92.5%) AMOX-treated patients and 47 (87.0%) PBO patients and poor in 3 (5.7%) AMOX-treated patients and 6 (11.1%) PBO patients (difference nonsignificant, p = 0.49). Twelve months after the end of intravenous antibiotic therapy, the levels of antibodies against Borrelia burgdorferi were markedly decreased in 50% of the patients with definite LB in both groups. The results indicate that oral adjunct antibiotics are not justified in the treatment of patients with disseminated LB who initially receive intravenous CRO for 3 weeks. The clinical outcome cannot be evaluated at the completion of intravenous antibiotic treatment but rather 6–12 months afterwards. In patients with chronic post-treatment symptoms, persistent positive levels of antibodies do not seem to provide any useful information for further care of the patient.





Similar content being viewed by others
References
Wormser GP, Dattwyler RJ, Shapiro ED et al (2006) The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134
Steere AC, Schoen RT, Taylor E (1987) The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731
Dinser R, Jendro MC, Schnar S, Zedler H (2005) Antibiotic treatment of Lyme borreliosis: what is the evidence? Ann Rheum Dis 64:519–523
Steere AC (2001) Lyme disease. N Engl J Med 345:115–125
Logigian EL, Kaplan RF, Steere AC (1990) Chronic neurologic manifestations of Lyme disease. N Engl J Med 323:1438–1444
Kristoferitsch W, Baumhackl U, Sluga E, Stanek G, Zeiler K (1987) High-dose penicillin therapy in meningopolyneuritis Garin–Bujadoux–Bannwarth. Clinical and cerebrospinal fluid data. Zentralbl Bakteriol Mikrobiol Hyg [A] 263:357–364
Ziska MH, Donta ST, Demarest FC (1996) Physician preferences in the diagnosis and treatment of Lyme disease in the United States. Infection 24:182–186
Wahlberg P, Granlund H, Nyman D, Panelius J, Seppälä I (1994) Treatment of late Lyme borreliosis. J Infect 29:255–261
Donta ST (1997) Tetracycline therapy for chronic Lyme disease. Clin Infect Dis 25(Suppl 1):52–56
Klempner MS, Hu LT, Evans J et al (2001) Two controlled trials of antibiotic treatment in patients with persistent symptoms, and a history of Lyme disease. N Engl J Med 345:85–92
Sigal LH (2002) Misconceptions about Lyme disease: confusions hiding behind ill-chosen terminology. Ann Intern Med 136:413–419
Stanek G, O’Connell S, Cimmino M et al (1996) European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr 108:741–747
Viljanen MK, Punnonen J (1989) The effect of storage of antigen-coated polystyrene microwells on the detection of antibodies against Borrelia burgdorferi by enzyme immunoassay (EIA). J Immunol Methods 124:137–141
Oksi J, Uksila J, Marjamäki M, Nikoskelainen J, Viljanen MK (1995) Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41 kilodalton flagellin and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J Clin Microbiol 33:2260–2264
Oksi J, Marttila H, Soini H, Aho H, Uksila J, Viljanen MK (2001) Early dissemination of Borrelia burgdorferi without generalized symptoms in patients with erythema migrans. APMIS 109:581–588
Halperin JJ, Luft BJ, Anand AK, Rogue CT, Alvarez O, Volkman DJ, Dattwyler RJ (1989) Lyme neuroborreliosis: central nervous system manifestations. Neurology 39:753–759
Dattwyler RJ, Wormser GP, Rush TJ et al (2005) A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien Klin Wochenschr 117:393–397
Baranton G, Postic D, Saint-Girons I et al (1992) Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS 461 associated with Lyme borreliosis. Int J Syst Bacteriol 42:378–383
van Dam AP, Kuiper H, Vos K et al (1993) Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis 17:708–717
Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V (2006) Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis 43:704–710
Krupp LB, Hyman LG, Grimson R et al (2003) Study and treatment of post Lyme disease (STOP-LD). A randomised double masked clinical trial. Neurology 60:1923–1930
Kaplan RF, Trevino RP, Johnson GM et al (2003) Cognitive function in post-treatment Lyme disease. Do additional antibiotics help? Neurology 60:1916–1922
Acknowledgements
The authors thank Bristol–Myers Squibb for their AMOX tablets and Roche for covering part of the costs of the study. The language of the manuscript was revised by Simo Merne, MA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oksi, J., Nikoskelainen, J., Hiekkanen, H. et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. Eur J Clin Microbiol Infect Dis 26, 571–581 (2007). https://doi.org/10.1007/s10096-007-0340-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0340-2