Abstract
An unusual case of cytomegalovirus (CMV) pneumonia in a diabetic patient is presented. The diagnosis was based on typical histopathological findings including intranuclear inclusion bodies combined with molecular identification of CMV in tissue specimens. The possibility of CMV reactivation associated with a previous cardiac procedure, which led to the development of usual interstitial pneumonia, is discussed. Clinicians should be aware of CMV-associated severe bilateral pneumonia developing after cardiac procedures even in non-transplant patients. The correct diagnosis depends on clinical awareness in the appropriate setting along with proof of viral infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventilator-associated pneumonia (VAP) due to cytomegalovirus (CMV) is a largely unexpected but probably underestimated diagnosis [1]. In one study, 25 of 86 patients with VAP and a prolonged stay in the intensive care unit (ICU) had CMV pneumonia [1]. The presence of CMV immunoglobulin (Ig)G antibodies may help identify patients susceptible to reactivation disease [1]. A recent report demonstrated that severely ill (i.e., SAPS II score, ≥41 points) ICU patients seropositive for CMV IgG antibodies frequently develop active CMV infection [2]. In that study, active infection progressed to severe and fatal disease in 10% of the cases examined [2]. Despite the fact that it is common to see CMV-associated interstitial lung disease in post-transplant patients [3], the existing literature contains only two cases in which CMV-related usual interstitial pneumonia occurred in patients with no transplant history [4, 5]. One report referred to a patient compromised by virtue of corticosteroid treatment [4] and the other patient was suffering from a concomitant thymoma [5]. Reported here is our experience with a diabetic patient who lacked any other apparent source of immunosuppression and developed usual interstitial pneumonia associated with CMV infection.
Case report
A 71-year-old woman was admitted to our hospital’s coronary care unit due to acute myocardial infarction. Her medical history included arterial hypertension, insulin-dependent diabetes mellitus, and hyperthyroidism. The patient had no history of tobacco or alcohol use. On admission, she was afebrile. Oxygen saturation was 97% on room air. Chest radiograph was normal. Blood glucose was 288 mg/dl.
She stayed in the coronary care unit for 48 h and was treated with aspirin (100 mg per day), clopidrogel (75 mg per day), heparin (1,000 IU/h), and a monoclonal antibody against platelet receptor Gp IIb/IIIa. Percutaneous transluminal coronary angioplasty (PTCA) was performed. Two days following discharge from the coronary care unit, the patient became febrile (38.5°C) with a non-productive cough and progressive dyspnea. Three days later she was admitted to the medical ICU due to severe dyspnea and type I respiratory failure. On admission, she had tachycardia (113 beats/min) and tachypnea (30 breaths/min). Her temperature was 38.6°C and arterial blood gas analysis disclosed a PaO2 of 58.0 mmHg, a PaCO2 of 25.8 mmHg, pH of 7.39, and a HCO3 of 15.6 mmol/l. Chest auscultation revealed end-inspiratory crackles, bilaterally diminished breath sounds, and a grade I apical systolic heart murmur. The patient was intubated. The chest radiograph taken at admission revealed diffuse infiltrates in both lungs and pleural effusions. A leukocyte count of 17,600 μl−1 (89% polymorphonuclear leukocytes) was noted, as well as a hematocrit level of 32%, a platelet count of 344,000 μl−1, and an erythrocyte sedimentation rate of 110 mm. Liver enzymes, and serum urea and creatinine were within normal limits.
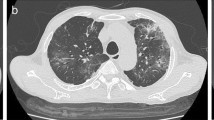
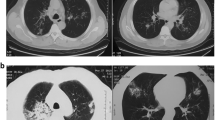
The patient was treated with intravenous piperacillin-tazobactam, ofloxacin and teicoplanin. Nevertheless, she remained febrile (39°C) and no improvement was noted in respiratory function and on chest radiograph. Blood, urine and bronchial cultures were negative for common bacterial and fungal pathogens. A computerized tomographic scan of the chest demonstrated confluent opacities in the right upper and middle lobes and in the left lower lobe as well as air-bronchograms; an extensive right pleural effusion and a pathological swelling of pretracheal lymph nodes were also noted.
The patient’s condition continued to deteriorate, and a thorough immunological evaluation was performed. Peripheral blood smear examination revealed no findings suggestive of asplenia. Tests to detect antinuclear antibodies, antineutrophil cytoplasmic antibodies against proteinase-3 and myeloperoxidase, and anti-DNA were all negative. Rheumatoid factor was normal, anti-smooth muscle antibodies were positive (titer, 1:20), and the C3 complement fraction was low. Plasma and urine protein electrophoresis did not reveal any monoclonal bands. Serologic tests for HIV and Legionella, Mycoplasma and Chlamydia species were negative. Tests were positive for IgG antibodies against CMV but no IgM antibodies were present.
Transthoracic echocardiography revealed mild mitral regurgitation, increased left atrial diameter, and normal left ventricular function. Pleural paracentesis revealed an exudate with 570 cells/μl (45% polymorphonuclear leukocytes and 55% lymphocytes), and Gram stain and cultures for common bacterial pathogens and fungi were negative. Bronchoscopy performed 25 days after admission revealed mucosal edema and erythema with no endobronchial mass present. Bronchoalveolar lavage (BAL) analysis was negative for common pathogens, fungi, Pneumocystis carinii, and Mycobacterium tuberculosis. A bronchial biopsy revealed mild inflammatory changes without evidence of any specific pathogen.
The patient’s ICU stay was complicated by the development of Enterobacter cloacae pneumonia and bacteremia on day 15. The isolated bacterial strain was sensitive to carbapenems and to ciprofloxacin. Additionally, she developed candiduria. The antibiotic regimen was modified to imipenem, ciprofloxacin, and vancomycin. Repeat Ziehl-Nielsen staining of tracheobronchial aspirates, BAL and pleural fluid analysis produced negative results. A new computed tomography scan of the chest showed similar findings to the first. The patient’s condition progressively worsened and she became hemodynamically unstable.
An open lung biopsy performed on day 55 of hospitalization revealed distortion of lung parenchyma, moderate inflammation and patchy fibrosis with a subpleural accentuation. The fibrotic areas consisted of dense collagen with a focal “honeycomb” pattern alternating with areas of relatively normal alveolar parenchyma. Also present were focal alveolar macrophage accumulation, smooth muscle proliferation and focal subpleural fatty metaplasia. The overall pattern was consistent with usual interstitial pneumonia. Some epithelial type II pneumocytes showed cytologic atypia with abundant cytoplasms and large pleomorphic nuclei harboring intranuclear inclusions consistent with CMV infection. Immunohistochemistry with antibodies against CMV (Signet Laboratories, Dedham, MA, USA) was positive in these cells (Fig. 1). Furthermore, PCR performed on DNA extracted from the paraffin-embedded tissue with CMV-specific primers showed a band of 110 bp, consistent with the presence of CMV-DNA. Despite treatment with intravenous ganciclovir (5 mg/kg every 12 h) the patient died on day 70 of hospitalization from severe acute respiratory distress syndrome and multiple organ failure.
Discussion
In the case presented here, we speculate that reactivation of CMV infection led to the development of usual interstitial pneumonia. One previous report has shown that many patients with pulmonary fibrosis exhibited CMV-specific IgG seropositivity and positive complement binding for CMV [6]. Previous studies have suggested that CMV is an atherogenic factor predisposing to coronary artery disease, and recent reports have stated that CMV contributed to one-third of restenosis cases following angioplasty [7, 8]. It has been hypothesized that CMV may be reactivated locally as a response to vascular injury in a subgroup of patients undergoing PTCA, and in some cases this may lead to severe infection [9]. In the present case, the possible association between previous PTCA and the subsequent development of fatal usual interstitial pneumonia associated with CMV is of extreme interest. It could be hypothesized that following myocardial infarction, elevated catecholamine titers may have led to CMV reactivation and transient CMV antigenemia, which eventually contributed to the patient’s lung injury. One recent report showed that catecholamine infusion or treatment with phosphodiesterase inhibitors, both of which increase c-AMP levels, could contribute to CMV reactivation [10].
An interesting aspect of this case is the diagnostic procedure used to identify the CMV infection. The detection of specific inclusion bodies in alveolar epithelial cells is the most reliable diagnostic criterion for CMV pneumonia. These bodies are rarely found in BAL fluid, because the virus preferentially attacks alveolar-endothelial and capillary-endothelial cells. For this reason, open lung biopsy is preferable to BAL testing and transbronchial biopsy. Although one could potentially diagnose idiopathic pulmonary fibrosis or diffuse interstitial lung disease based on clinical and radiographic criteria, in most cases it is necessary to perform surgical lung biopsy for the diagnosis and to determine the underlying histopathology [11]. Analysis of BAL specimens using CMV-specific PCR measurement of viral load may contribute to the diagnosis if open lung biopsy is not feasible [12].
CMV pneumonia must be included in the differential diagnosis in the appropriate clinical setting. Even among patients presenting with community-acquired pneumonia Marrie et al. [13] found that 4 of 443 (0.9%) patients had documented CMV pneumonia as the underlying diagnosis. Another 14 of 443 (3%) patients had a fourfold increase in CMV IgM titers. Among these patients, only six were immunocompromised, seven needed mechanical ventilation and five died [13]. In hospitalized patients, CMV is usually transmitted by means of blood products and via transplanted organs [14]. In the case presented here, we did not find evidence of recently acquired acute CMV infection. In addition there was no history of recent transfusion or overt immunosuppression, except for the diagnosis of diabetes mellitus. In a search of the medical literature we did not find reports of increased incidence of CMV infection in diabetic patients, although diabetic patients do have an increased susceptibility to infection.
In the case of our diabetic patient with CMV-associated usual interstitial pneumonia, it is likely that the initial pulmonary infection was due to a bacterial cause, and that CMV was a pathogen that caused infection at a later time during the course of hospitalization. The isolation of E. cloacae in the bronchial secretions of our patient late during her ICU stay may represent coinfection or superinfection, both of which commonly occur during CMV infection. This could be due to several immunomodulating properties of CMV [2, 15]. The correct diagnosis of CMV-associated severe bilateral pneumonia, even in patients who are not overtly immunocompromised, depends on clinical awareness in the appropriate setting along with definitive proof of viral infection. Even in debilitated patients without risk factors, such as advanced HIV infection or iatrogenic immunosuppression, CMV may be an underestimated cause of pneumonia since it occurs in patients who have undergone transplantation or who have a hematologic malignancy.
References
Papazian L, Fraisse A, Garbe L et al (1996) CMV. An unexpected cause of VAP. Anesthesiology 84:280–287
Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K (2001) Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med 29:541–547
Falagas ME, Snydman DR, George MI et al (1996) Incidence and predictors of cytomegalovirus pneumonia in orthotopic liver transplant recipients. Transplantation 61:1716–1720
Tokimatsu I, Tashiro T, Ichimiya T et al (1992) A case of idiopathic interstitial pneumonia with cytomegalovirus infection (article in Japanese). Nihon Kyobu Shikkan Gakkai Zasshi 30:941–946
Yonemaru M, Ustumi K, Kasuga I et al (1994) A case of pulmonary fibrosis associated with CMV inclusion body (article in Japanese). Nihon Kyobu Shikkan Gakkai Zasshi 32:184–188
Yonemaru M, Kasuga I, Kusumoto H et al (1997) Elevation of antibodies to CMV and other herpes viruses in pulmonary fibrosis. Eur Respir J 10:2040–2045
Zhou YF, Leon MB, Waclawiw MA et al (1996) Association between prior cytomegalovirus infection and the risk of re-stenosis after coronary atherectomy. N Engl J Med 335:624–630
Streblow DN, Orloff SL, Nelson JA (2001) Do pathogens accelerate atherosclerosis? J Nutr 131:2798–2804
Salomon N, Perlman DC (1999) CMV pneumonia. Semin Respir Infect 14:353–358
Prosch S, Wendt CE, Reinke P et al (2000) A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272:357–365
Collard HR, King TE Jr (2003) Demystifying idiopathic interstitial pneumonia. Arch Intern Med 163:17–29
Kjellstrom C, Bergstrom T, Martensson G et al (1997) Relation between PCR findings and morphological changes during CMV infection in transplanted lung. Diagn Mol Pathol 6:267–276
Marrie TJ, Janigan DT, Haldane EV, Faulkner RS, Kwan C, Durant H (1985) Does CMV play a role in community-acquired pneumonia? Clin Invest Med 8:286–295
Barbera JA, Martin-Campos JM, Ribalta T et al (1996) Undetected viral infection in diffuse alveolar damage associated with bone marrow transplantation. Eur Respir J 9:1195–2000
Falagas ME, Snydman DR, Griffith J, Werner BG (1996) Exposure to cytomegalovirus from the donated organ is a risk factor for bacteremia in orthotopic liver transplant recipients. Clin Infect Dis 23:468–474
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizos, M., Falagas, M.E., Tsiodras, S. et al. Usual interstitial pneumonia associated with cytomegalovirus infection after percutaneous transluminal coronary angioplasty. Eur J Clin Microbiol Infect Dis 23, 848–850 (2004). https://doi.org/10.1007/s10096-004-1220-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-004-1220-7