Abstract
In order to detect immunoglobulin (Ig)A and IgG antibodies to Escherichia coli-secreted protein B in sera of children infected with Shiga toxin-producing Escherichia coli, an enzyme-linked immunosorbent assay was developed. The assay was tested using acute sera from 40 children with diarrhea-associated hemolytic uremic syndrome compared with 238 sera obtained from pediatric controls. Two cut-off values were used for children <5 (n=27) or ≥5 (n=13) years of age. Among the younger patients, 24 of 27 had IgA antibodies to Escherichia coli-secreted protein B (sensitivity, 89%; specificity, 98%) and 22 of 27 had IgG antibodies (sensitivity, 82%; specificity, 94%). Among the older patients, 13 of 13 had IgA antibodies (sensitivity, 100%; specificity, 96%) and 11 of 13 had IgG antibodies (sensitivity, 85%; specificity, 96%). This enzyme-linked immunosorbent assay detects Shiga-toxin-producing Escherichia coli independent of serogroup and could serve as a complementary assay for detection of infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shiga toxin (Stx)-producing Escherichia coli (STEC) cause diarrhea, hemorrhagic colitis and hemolytic uremic syndrome (HUS) [1]. The most prevalent serotype is Escherichia coli O157:H7, but more than 100 serotypes have been associated with clinical symptoms [1]. Following ingestion, the bacteria colonize the intestine forming an “attaching and effacing” lesion on the intestinal epithelium [2, 3]. EspB is a polypeptide of 37 kDa involved in signal transduction events occurring during the formation of the attaching and effacing lesion [4].
Diagnosis of STEC infection is predominantly based on the isolation and serotyping of a STEC strain from the feces of infected individuals. Fecal strains are also identified by polymerase chain reaction (PCR) amplification of virulence genes such as stx and eae in fecal samples or by the detection of free Stx in the stool [3]. Serodiagnosis is based on the detection of serum antibodies to the lipopolysaccharide of the infecting strain and is serogroup dependent [5, 6, 7]. Serological diagnostic methods may also be based on the presence of antibodies to Escherichia coli-secreted proteins [8, 9, 10].
In a previous study we developed a serodiagnostic immunoblotting assay for the detection of STEC infection based on the presence of antibodies to recombinant EspB (from an Escherichia coli O157:H7 template) in the sera of infected individuals [11]. This assay is serogroup independent and facilitates the detection of STEC infection in a clinical setting of hemorrhagic colitis or HUS in which the bacterial strain could not be isolated from feces. The aim of the present study was to develop an enzyme-linked immunosorbent assay (ELISA) for the detection of serum antibodies to EspB in HUS patients with STEC infection.
Materials and Methods
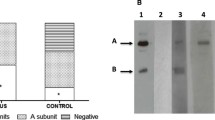
The espB construct used in this study was amplified using Escherichia coli O157:H7 strain 93111 as the template and the His-EspB fusion protein, which was purified from Escherichia coli DH5a(pREP4,pCVD469) as described previously [11]. The antiserum against His-EspB was raised in rabbits and reacts with His-EspB in both immunoblotting and ELISA.
Sera obtained from 40 pediatric patients during the acute phase of HUS were analyzed. There were 17 boys and 23 girls aged 1–11 years (median age, 3 years). Twenty-seven of the children were <5 years old (median, 2 years) and 13 children were ≥5 years old (median, 7 years). All of the children had clinical evidence of STEC infection due to prodromal diarrhea. All patients were treated at the Department of Pediatrics, Lund University Hospital. Samples were collected on the day of admission.
The infecting strain was detected by PCR identification of the eae and stx genes in feces followed by isolation of a bacterial strain, which was serotyped [11, 12]. Fecal samples were available in 33 cases, and in two of these cases PCR was negative. In these cases, and when a fecal STEC strain was unavailable, serum (n=9) was tested for antibodies to the lipopolysaccharide of serogroups O157, O26, O103, O111 and O145 as described previously [6]. In four cases serum was also tested for antibodies to the lipopolysaccharide of serogroups O91, O113 and O121. When a strain was not isolated or the serological assay was negative, the presence of STEC was assumed, due to a diarrheal prodrome followed by HUS, but not proven. Thus, infection was associated with Escherichia coli O157 in 20 cases, with other non-O157 STEC serogroups (n=16) or not proven (n=4). Thirty-two of the 40 patients with HUS were described previously [11].
Control sera were obtained from 238 pediatric controls (125 boys and 113 girls; age range, <1–18 years; median age, 5 years). Of these children, 117 were <5 years old (median, 1 year) and 121 were ≥5 years old (median, 10 years). The pediatric controls did not have a history of diarrhea or HUS. Samples were stored at −20°C until assayed.
Serum antibody responses to EspB were assayed using the newly developed ELISA. Microtiter plates were coated with rabbit anti His-EspB 1/2000 (100 µl/well in 0.1 M NaHCO3, pH 9.6) and incubated overnight at 4°C. Wells were washed in PBS/Tween 0.05% (Medicago, Uppsala, Sweden), blocked for 2 h with 3% bovine serum albumin (BSA; ICN Biomedicals, Aurora, OH, USA) in PBS/Tween at room temperature, washed, and incubated for 1 h at room temperature with recombinant His-EspB (50 ng/well in 0.1% BSA/PBS/Tween) [11]. Wells were washed and incubated with patient or control sera (1/50 in 0.1% BSA/PBS/Tween) at a total volume of 100 µl/well for 2 h at 37°C. After washing, peroxidase-conjugated rabbit anti-human immunoglobulin (1/2000 for anti-IgA or 1/4000 for anti-IgG) (antibodies from Dako, Glostrup, Denmark) in 100 µl, were added and incubated for 1.5 h at room temperature. Binding was detected by the addition of O-phenylendiamine dihydrochloride tablets (Dako) suspended in deionized water for 10 min at room temperature in the dark, then 30% H2O2 (Scharlau Chemie, Barcelona, Spain) was added and the reaction was terminated by the addition of 1 M H2SO4 (Merck, Darmstadt, Germany). Absorbance was measured at OD450 nm.
Since the antibody response normally increases with age and most children with HUS are <5 years old, cut-off values were established for children <5 years of age and ≥5 years of age. Using the sera from the 238 pediatric controls, a cut-off value was assigned for each age group and antibody type by the mean value +2 standard deviations. A value above this cut-off was considered positive. Differences between patients and controls regarding a specific cut-off value were evaluated with Fisher’s exact test using SPSS version 11.0 (SPSS, Chicago, IL, USA). A P value <0.05 was considered significant. Sensitivity and specificity values for the different antibodies were compared.
Results and Discussion
Nearly all of the sera from the 40 HUS patients exhibited an IgA and IgG response to EspB, while nearly all of the sera from controls did not. For the IgA response, a cut-off value of 0.18 was assigned for patients aged <5 years and 0.25 for those aged ≥5 years. For the IgG response, a cut-off value of 0.89 was assigned for patients <5 years of age and 1.24 for those aged ≥5 years. Using these cut-off values, 24 of the 27 patients <5 years of age had an IgA response versus 2 of 117 controls (P<0.0005) and 13 of 13 patients ≥5 years of age had an IgA response versus 5 of 121 controls (P<0.0005). An IgG response was detected in 22 of 27 children <5 years of age versus 6 of 117 controls (P<0.0005) and in 11 of 13 children aged ≥5 years versus 5 of 121 controls (P<0.0005). Immunoglobulin A and IgG antibodies to EspB were found in patients with O157 and non-O157 infection as shown in Table 1, and are thus serogroup independent.
The sensitivity and specificity of the ELISA for detecting IgA and IgG antibodies to EspB in sera of patients with HUS are shown in Table 2. These results show that higher sensitivity values were obtained for IgA antibodies to EspB than IgG in both age groups, and the specificity values for both antibodies was ≥94%.
The results of this study demonstrate that a considerable proportion of pediatric patients with HUS associated with acute STEC infection mount an antibody response to EspB, which can be readily detected by ELISA. The antibody response is independent of the STEC serogroup and is thus appropriate for the detection of both O157 and non-O157 STEC infections. Enteropathogenic Escherichia coli strains also produce EspB. There is considerable sequence homology between EspB of different STEC and enteropathogenic Escherichia coli strains [4, 11], and patients with enteropathogenic Escherichia coli infection have been found to have antibodies to EspB [13]. Thus, this assay may not differentiate between patients with milder STEC symptoms (e.g., non-bloody diarrhea, non-HUS) and those infected with enteropathogenic Escherichia coli. The presence of antibodies to EspB in patient serum will, however, indicate STEC infection in the clinical context of HUS, which is not associated with enteropathogenic Escherichia coli.
Serodiagnosis of STEC infection is currently achieved by the detection of antibodies to the lipopolysaccharide of a given serogroup by ELISA. This assay has high sensitivity (92–95%) and specificity (97–99%) when patients have culture-confirmed Escherichia coli O157 infection [14, 15], but not all patients with confirmed Escherichia coli O157 infection mount an antibody response to the O157 lipopolysaccharide [15]. Moreover, it is serogroup-dependent and lipopolysaccharide from other STEC serogroups have been added to the O157 lipopolysaccharide assay [6, 7]. Using a panel of five serogroups (O157, O26, O103, O111 and O145) the assay provided evidence of STEC infection in 61.3% of 235 patients with HUS in Italy [6] and using a panel of 26 serogroups the assay detected 74% of 118 HUS patients in France [7]. The panel of appropriate STEC serogroups to be tested may vary according to the epidemiological situation of different geographical areas.
In this study, patients infected with Escherichia coli O157 and with at least four other locus of enterocyte effacement-positive serogroups developed antibodies to the EspB of STEC O157. We therefore suggest that this ELISA for the detection of EspB can be an efficient serogroup-independent assay for the diagnosis of STEC infection as long as the infecting STEC strain is locus of enterocyte effacement-positive. Laboratory diagnosis of STEC infection requires a combination of microbiological and serological techniques. The development of an ELISA for the detection of EspB will serve as a valuable adjunct to available methods for the detection of STEC infection.
References
Griffin PM (1995) Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL (eds) Infections of the gastrointestinal tract. Raven Press, New York, pp 739–761
Jarvis KG, Giron JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB (1995) Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA 92:7996–8000
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201
Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S (1998) Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol 30:911–921
Chart H, Jenkins C (1999) The serodiagnosis of infections caused by verocytotoxin-producing E. coli. J Appl Microbiol 86:731–740
Tozzi AE, Caprioli A, Minelli F, Gianviti A, De Petris L, Edefonti A, Montini G, Ferretti A, De Palo T, Gaido M, Rizzoni G; Hemolytic Uremic Syndrome Study Group (2003) Shiga toxin-producing Escherichia coli infections associated with hemolytic uremic syndrome, Italy, 1988–2000. Emerg Infect Dis 9:106–108
Decludt B, Bouvet P, Mariani-Kurkdjian P, Grimont F, Grimont PA, Hubert B, Loirat C (2000) Haemolytic uraemic syndrome and Shiga toxin-producing Escherichia coli infection in children in France. The Societe de Nephrologie Pediatrique. Epidemiol Infect 124:215–220
Jarvis KG, Kaper JB (1996) Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect Immun 64:4826–4829
Li Y, Frey E, Mackenzie AM, Finlay BB (2000) Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect Immun 68:5090–5095
Jenkins C, Chart H, Smith HR, Hartland EL, Batchelor M, Delahay RM, Dougan G, Frankel G (2000) Antibody response of patients infected with verocytotoxin-producing Escherichia coli to protein antigens encoded on the LEE locus. J Med Microbiol 49:97–101
Karpman D, Bekassy ZD, Sjogren AC, Dubois MS, Karmali MA, Mascarenhas M, Jarvis KG, Gansheroff LJ, O’Brien AD, Arbus GS, Kaper JB (2002) Serum antibodies to intimin and secreted proteins in patients with Escherichia coli O157:H7 infections. Pediatr Nephrol 17:201–211
Svenungsson B, Lagergren A, Ekwall E, Evengard B, Hedlund KO, Karnell A, Lofdahl S, Svensson L, Weintraub A (2000) Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin Infect Dis 30:770–778
Martinez MB, Taddei CR, Ruiz-Tagle A, Trabulsi LR, Girón JA (1999) Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J Infect Dis 179:269–274
Ludwig K, Bitzan M, Bobrowski C, Muller-Wiefel DE (2002) Escherichia coli O157 fails to induce a long-lasting lipopolysaccharide-specific, measurable humoral immune response in children with hemolytic-uremic syndrome. J Infect Dis 186:566–569
Barrett TJ, Green JH, Griffin PM, Pavia AT, Ostroff SM, Wachsmuth IK (1991) Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, and Escherichia coli O157:H7 lipopolysaccharide in human serum. Curr Microbiol 23:189–195
Acknowledgements
We thank Dr. Lennart Truedsson, Department of MIG, Lund University, for supplying most of the pediatric control sera. The statistical assistance of Vibeke Horstmann is appreciated. This study was supported by grants from The Swedish Research Council (06X-14008), the Swedish Renal Foundation, the Åke Wiberg Foundation, the Anna-Lisa and Sven-Eric Lundgren Foundation for Medical Research, the Ronald McDonald Pediatric Fund, the Sven Jerring Foundation, the Greta and Johan Kock Foundation, the Swedish Society of Medicine, the Crafoord foundation, the Inga and John Hains foundation, the Royal Physiographic Society in Lund, the Alfred Österlund Foundation, the Thelma Zoegas foundation, The Swedish Society of Nephrology, Skånska Provinsiallogens Welfare Fund and the Lund University Hospital funds (all to DK) and NIH grants AI41324 and DK58957 (both to JBK). The experiments carried out in this study comply with the current laws of Sweden, and the study was approved by the ethics committee of Lund University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sjögren, AC., Kaper, J.B., Caprioli, A. et al. Enzyme-Linked Immunosorbent Assay for Detection of Shiga Toxin-Producing Escherichia coli Infection by Antibodies to Escherichia coli Secreted Protein B in Children with Hemolytic Uremic Syndrome. Eur J Clin Microbiol Infect Dis 23, 208–211 (2004). https://doi.org/10.1007/s10096-003-1090-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-003-1090-4