Abstract
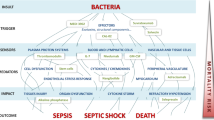
Excessive production of inflammatory mediators during invasive infection plays a key role in the pathogenesis of septic shock. In an attempt to improve survival of patients with this lethal syndrome, agents were developed to selectively inhibit mediators in this inflammatory response. Despite promising preclinical results, several different mediator-specific anti-inflammatory agents failed to demonstrate significant benefit in patients. There was, however, a significant difference in mortality between preclinical and clinical trials. The median control mortality in preclinical trials, performed almost uniformly in highly lethal sepsis models, was 88%. In clinical trials however, the median control mortality rate was much lower, at 41%. A recent meta-regression analysis of these preclinical and clinical trials in combination with prospective confirmatory studies demonstrated that risk of death as assessed by control group mortality rate significantly altered the treatment effect of these agents in both humans and animals. While anti-inflammatory agents were very beneficial in groups with high control mortality rates, they were ineffective or harmful in groups with low control mortality rates. Thus, variation in the risk of death due to sepsis provides a basis for the marked difference in the efficacy of these anti-inflammatory agents in preclinical and clinical trials over the last decade. In contrast to mediator-specific anti-inflammatory agents, glucocorticoids and activated protein C have recently demonstrated significant beneficial effects in individual clinical trials. However, glucocorticoids were studied only in patients with vasopressor-dependent septic shock, which is associated with a high control mortality rate (i.e. 61%) similar to the level at which mediator-specific agents would have been expected to be markedly beneficial. Furthermore, consistent with earlier findings for mediator-specific anti-inflammatory agents, analysis of the activated protein C study also demonstrated a relationship between risk of death and effect of treatment. Developing better methods to define high-risk septic populations for treatment with anti-inflammatory agents will increase the efficacy of this therapeutic approach and minimize its potential for harm.





Similar content being viewed by others
References
Centers for Disease Control (1990) Increase in national hospital discharge survey rates for septicemia – United States, 1979–1987. MMWR 39:31–34
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Jimenez MF, Marshall JC (2001) Source control in the management of sepsis. Intensive Care Med 27 [Suppl 1]:49–62
Natanson C, Danner RL, Reilly JM, Doerfler ML, Hoffman WD, Akin GL, Hosseini JM, Banks SM, Elin RJ, MacVittie TJ, Parillo JE (1990) Antibiotics versus cardiovascular support in a canine model of human septic shock. Am J Physiol 259:H1440–1447
Natanson C, Hoffman WD, Suffredini AF, Eichacker PQ, Danner RL (1994) Selected treatment strategies for septic shock based on proposed mechanisms of pathogenesis. Ann Intern Med 120:771–783
Zeni F, Freeman B, Natanson C (1997) Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 25:1095–1100
Quezado ZM, Banks SM, Natanson C (1995) New strategies for combatting sepsis: the magic bullets missed the mark ... but the search continues. Trends Biotechnol 13:56–63
Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE (1989) The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 321:280–287
Alexander RB, Ponniah S, Hasday J, Hebel JR (1998) Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology 52:744–749
Natanson C, Eichenholz PW, Danner RL, Eichacker PQ, Hoffman WD, Kuo GC, Banks SM, MacVittie TJ, Parrillo JE (1989) Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med 169:823–832
Waage A, Espevik T (1988) Interleukin 1 potentiates the lethal effect of tumor necrosis factor alpha/cachectin in mice. J Exp Med 167:1987–1992
Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ III, Zentella A, Albert JD, Shires GT, Cerami A (1986) Shock and tissue injury induced by recombinant human cachectin. Science 234:470–474
Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA (1988) Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest 81:1162–1172
Eichacker PQ, Hoffman WD, Farese A, Banks SM, Kuo GC, MacVittie TJ, Natanson C (1991) TNF but not IL-1 in dogs causes lethal lung injury and multiple organ dysfunction similar to human sepsis. J Appl Physiol 71:1979–1989
Waage A, Halstensen A, Espevik T (1997) Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet I:355–357
Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC (1990) Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348:550–552
Wakabayashi G, Gelfand JA, Burke JF, Thompson RC, Dinarello CA (1991) A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J 5:338–343
Fischer E, Marano MA, Van Zee KJ, Rock CS, Hawes AS, Thompson WA, DeForge L, Kenney JS, Remick DG, Bloedow DC, Thompson RC, Lowry SF, Moldawer LL (1992) Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host responses to sublethal endotoxemia. J Clin Invest 89:1551–1557
Beutler B, Milsark IW, Cerami AC (1985) Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229:869–871
Suitters AJ, Foulkes R, Opal SM, Palardy JE, Emtage JS, Rolfe M, Stephens S, Morgan A, Holt AR, Chaplin LC, et al (1994) Differential effect of isotype on efficacy of anti-tumor necrosis factor alpha chimeric antibodies in experimental septic shock. J Exp Med 179:849–856
Bagby GJ, Plessala KJ, Wilson LA, Thompson JJ, Nelson S (1991) Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis 163:83–88
Mathison JC, Wolfson E, Ulevitch RJ (1988) Participation of tumor necrosis factor in the mediation of gram-negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest 81:1925–1937
Fiedler VB, Loof I, Sander E, Voehringer V, Galanos C, Fournel MA (1992) Monoclonal antibody to tumor necrosis factor-alpha prevents lethal endotoxin sepsis in adult rhesus monkeys. J Lab Clin Med 120:574–588
Emerson TE Jr, Lindsey DC, Jesmok GJ, Duerr ML, Fournel MA (1992) Efficacy of monoclonal antibody against tumor necrosis factor-alpha in an endotoxemic baboon model. Circ Shock 38:75–84
Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG (1992) Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 148:2724–2730
Silva AT, Bayston KF, Cohen J (1990) Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis 162:421–427
Jesmok G, Lindsey C, Duerr M, Fournel M, Emerson T Jr (1992) Efficacy of monoclonal antibody against human recombinant tumor necrosis factor in E. coli-challenged swine. Am J Pathol 141:1197–1207
Hinshaw LB, Tekamp-Olson P, Chang AC, Lee PA, Taylor FB Jr, Murray CK, Peer GT, Emerson TE Jr, Passey RB, Kuo GC (1990) Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF-alpha). Circ Shock 30:279–292
Hinshaw LB, Emerson TE Jr, Taylor FB Jr, Chang AC, Duerr M, Peer GT, Flournoy DJ, White GL, Kosanke SD, Murray CK, Xu R, Passey RB, Fournei MA (1992) Lethal Staphylococcus aureus-induced shock in primates: prevention of death with anti-TNF antibody. J Trauma 33:568–573
Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330:662–664
Opal SM, Cross AS, Kelly NM, Sadoff JC, Bodmer MW, Palardy JE, Victor GH (1990) Efficacy of a monoclonal antibody directed against tumor necrosis factor in protecting neutropenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis 161:1148–1152
Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C (2002) Risk and efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 166:1197–1205
Wheeler AP, Bernard GR (1999) Treating patients with severe sepsis. N Engl J Med 340:207–214
Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328:1471–1477
Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M (2001) Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study. Crit Care Med 29:765–769
Kay C (1996) Reducing mortality to patients and suppliers. In: Proceedings of the Cambridge Health Institute conference "Designing Better Drugs and Clinical Trials for Sepsis/SIRS," Washington DC
Sevransky J, Natanson C (1999) Published clinical trials in sepsis: an update. Sepsis 2:11–19
Reinhart K, Wiegand-Lohnert C, Grimminger F, Kaul M, Withington S, Treacher D, Eckart J, Willatts S, Bouza C, Krausch D, Stockenhuber F, Eiselstein J, Daum L, Kempeni J (1996) Assessment of the safety and efficacy of the monoclonal anti-tumor necrosis factor antibody-fragment, MAK 195F, in patients with sepsis and septic shock: a multicenter, randomized, placebo-controlled, dose-ranging study. Crit Care Med 24:733–742
Fisher CJ Jr, Opal SM, Dhainaut JF, Stephens S, Zimmerman JL, Nightingale P, Harris SJ, Schein RM, Panacek EA, Vincent JL, et al (1993) Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. Crit Care Med 21:318–327
Dhainaut JF, Vincent JL, Richard C, Lejeune P, Martin C, Fierobe L, Stephens S, Ney UM, Sopwith M (1995) CDP571, a humanized antibody to human tumor necrosis factor-alpha: safety, pharmacokinetics, immune response, and influence of the antibody on cytokine concentrations in patients with septic shock. Crit Care Med 23:1461–1469
Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R, Pennington JE, Wherry JC (1995) Efficacy and safety of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. JAMA 273:934–941
Cohen J, Carlet J (1996) INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. Crit Care Med 24:1431–1440
Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, Levy H, Baughman R, Rumbak M, Light RB, Poole L, Allred R, Constant J, Pennington J, Porter S (1998) Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 351:929–933
Clark MA, Plank LD, Connolly AB, Streat SJ, Hill AA, Gupta R, Monk DN, Shenkin A, Hill GL (1998) Effect of a chimeric antibody to tumor necrosis factor-alpha on cytokine and physiologic responses in patients with severe sepsis – a randomized, clinical trial. Crit Care Med 26:1650–1659
Fisher CJ Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, Ng D, Bloedow DC, Catalano MA (1994) Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med 22:12–21
Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 25:1115–1124
Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al (1994) Recombinant human interleukin-1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. JAMA 271:1836-1843
Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, Higgins SB, Swindell BB (1997) The effects of ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med 336:912–918
Bernard GR, Reines HD, Halushka PV, Higgins SB, Metz CA, Swindell BB, Wright PE, Watts FL, Vrbanac JJ (1991) Prostacyclin and thromboxane A2 formation is increased in human sepsis syndrome. Effects of cyclooxygenase inhibition. Am Rev Respir Dis 144:1095–1101
Haupt MT, Jastremski MS, Clemmer TP, Metz CA, Goris GB (1991) Effect of ibuprofen in patients with severe sepsis: a randomized, double-blind, multicenter study. Crit Care Med 19:1339–1347
Rodell TC, Scharschmidt L, Knaus WA (1995) CP-0127 SIRS and Sepsis Study Group: results of a multi-center randomized, placebo-controlled trial of CP-0127, a novel bradykinin antagonist, in patients with SIRS and sepsis. Shock 3:60
Fein AM, Bernard GR, Criner GJ, Fletcher EC, Good JT Jr, Knaus WA, Levy H, Matuschak GM, Shanies HM, Taylor RW, Rodell TC (1997) Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. JAMA 277:482–487
Dhainaut JF, Tenaillon A, Hemmer M, Damas P, Le Tulzo Y, Radermacher P, Schaller MD, Sollet JP, Wolff M, Holzapfel L, Zeni F, Vedrinne JM, de Vathaire F, Gourlay ML, Guinot P, Mira JP (1998) Confirmatory platelet-activating factor receptor antagonist trial in patients with severe gram-negative bacterial sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 26:1963–1971
Dhainaut JF, Tenaillon A, Le Tulzo Y, Schlemmer B, Solet JP, Wolff M, Holzapfel L, Zeni F, Dreyfuss D, Mira JP, De Vathaire F, Guinot P (1994) Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med 22:1720–1728
Freeman BD, Natanson C (2000) Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs 9:1651–1663
Knaus WA, Harrell FE Jr, LaBrecque JF, Wagner DP, Pribble JP, Draper EA, Fisher CJ Jr, Soll L (1996) Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. Crit Care Med 24:46–56
Abraham E, Laterre PF, Garbino J, Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli W, Anderson P, Reynaert M, Lew D, Lesslauer W, Passe S, Cooper P, Burdeska A, Modi M, Leighton A, Salgo M, Van der Auwera P (2001) Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit Care Med 29:503–510
Reinhart K, Menges T, Gardlund B, Harm-Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M (2001) Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES study. Crit Care Med 29:765–769
Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E (1996) Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med 334:1697–1702
Abraham E, Glauser MP, Butler T, Garbino J, Gelmont D, Laterre PF, Kudsk K, Bruining HA, Otto C, Tobin E, Zwingelstein C, Lesslauer W, Leighton A (1997) p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. JAMA 277:1531–1538
Fabian TC, Patterson R (1982) Steroid therapy in septic shock. Survival studies in a laboratory model. Am Surg 48:614–617
Hinshaw LB, Beller-Todd BK, Archer LT, Benjamin B, Flournoy DJ, Passey R, Wilson MF (1981) Effectiveness of steroid/antibiotic treatment in primates administered LD100 Escherichia coli. Ann Surg 194:51–56
Hinshaw LB, Archer LT, Beller-Todd BK, Benjamin B, Flournoy DJ, Passey R (1981) Survival of primates in lethal septic shock following delayed treatment with steroid. Circ Shock 8:291–300
Hinshaw LB, Beller BK, Archer LT, Flournoy DJ, White GL, Phillips RW (1979) Recovery from lethal Escherichia coli shock in dogs. Surg Gynecol Obstet 149:545–553
Hinshaw LB, Archer LT, Beller-Todd BK, Coalson JJ, Flournoy DJ, Passey R, Benjamin B, White GL (1980) Survival of primates in LD100 septic shock following steroid/antibiotic therapy. J Surg Res 28:151–170
White GL, Archer LT, Beller BK, Hinshaw LB (1978) Increased survival with methylprednisolone treatment in canine endotoxin shock. J Surg Res 25:357–364
Beller BK, Archer LT, Passey RB, Flournoy DJ, Hinshaw LB (1983) Effectiveness of modified steroid-antibiotic therapies for lethal sepsis in the dog. Arch Surg 118:1293–1299
Lucas CE, Ledgerwood AM (1984) The cardiopulmonary response to massive doses of steroids in patients with septic shock. Arch Surg 119:537–541
Schumer W (1976) Steroids in the treatment of clinical septic shock. Ann Surg 184:333–341
Klastersky J, Cappel R, Debusscher L (1971) Effectiveness of betamethasone in management of severe infections. A double-blind study. N Engl J Med 284:1248–1250
Bennett IL, Finland M, Hamborger M, Kass EH, Lepper M, Waisbren BA (1963) The effectiveness of hydrocortisone in the management of severe infection. JAMA 183:462–465
Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF (1988) Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 138:62–68
The Veterans Administration Systemic Sepsis Cooperative Study Group (1987) Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. N Engl J Med 317:659–665
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1987) A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med 317:653–658
Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, Duncan RC, Tendler MD, Karpf M (1984) The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med 311:1137–1143
Rothwell PM, Udwadia ZF, Lawler PG (1991) Cortisol response to corticotropin and survival in septic shock. Lancet 337:582–583
Moran JL, Chapman MJ, O'Fathartaigh MS, Peisach AR, Pannall PR, Leppard P (1994) Hypocortisolaemia and adrenocortical responsiveness at onset of septic shock. Intensive Care Med 20:489–495
Soni A, Pepper GM, Wyrwinski PM, Ramirez NE, Simon R, Pina T, Gruenspan H, Vaca CE (1995) Adrenal insufficiency occurring during septic shock: incidence, outcome, and relationship to peripheral cytokine levels. Am J Med 98:266–271
Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A (1998) Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 26:645–650
Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, Stoll C, Peter K (1999) Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 27:723–732
Chawla K, Kupfer Y, Goldman I, Tessler S (1999) Hydrocortisone reverses refractory shock. Crit Care Med 27 [Suppl 1]:A33
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach J, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellisant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871
Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA (1989) Sepsis syndrome: a valid clinical entity. Crit Care Med 17:389–393
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Minneci, P., Deans, K., Natanson, C. et al. Increasing the Efficacy of Anti-Inflammatory Agents Used in the Treatment of Sepsis. Eur J Clin Microbiol Infect Dis 22, 1–9 (2003). https://doi.org/10.1007/s10096-002-0857-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-002-0857-3