Abstract
The main aim of this study was to investigate the efficacy of a dual task protocol in people with episodic migraine with respect to both active exercises only and cognitive task only treatments, concerning some neurophysiological and clinical outcomes. A randomized control trial was adopted in people with episodic migraine without aura. Some neurophysiological and clinical outcomes were collected (t0): resting motor threshold (rMT), short intracortical inhibition (SICI) and facilitation (ICF), pressure pain threshold (PPT), trail making test (TMT), frontal assessment battery (FAB), headache-related disability (MIDAS) and headache parameters. Then, participants were randomized into three groups: active exercise only (n = 10), cognitive task only (n = 10) and dual task protocol (n = 10). After 3 months of each treatment and after 1-month follow-up the same neurophysiological and clinical outcomes were revaluated. A significant time x group effect was only found for the trapezius muscle (p = 0.012, pη2 = 0.210), suggesting that PPT increased significantly only in active exercise and dual task protocol groups. A significant time effect was found for rMT (p < 0.001, pη2 = 0.473), MIDAS (p < 0.001, pη2 = 0.426), TMT (p < 0.001, pη2 = 0.338) and FAB (p < 0.001, pη2 = 0.462). A repeated measures ANOVA for SICI at 3 ms highlighted a statistically significant time effect for the dual task group (p < 0.001, pη2 = 0.629), but not for the active exercises group (p = 0.565, pη2 = 0.061), and for the cognitive training (p = 0.357, pη2 = 0.108). The dual task protocol seems to have a more evident effect on both habituation and sensitization outcomes than the two monotherapies taken alone in people with migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine represents the most common neurologic condition with an important socioeconomic burden, particularly for Italy, but also for many other countries. Part of migraine burden is due to its complex physiopathology that is still object of studies for a better understanding of the underlying mechanisms and, as a consequence, for a better choice of the related treatments and clinical management [1, 2]. Concerning physiopathology, researchers defined migraine “a brain state of altered excitability”[3] in a “migraine brain”. In fact, neurophysiological studies highlighted in people with migraine a general neuronal hyper-responsivity to innocuous sensory and noxious stimuli [4, 5], especially between attacks. This phenomenon may depend on two opposing processes: lack of habituation and sensitization that determine alteration in the sensory processing[3,4,5,6,7,8]. Habituation represents an inhibitory response to sensory stimulation. Sensitization represents an augmentation response to sensory stimulation[5]. On one hand, lack of habituation is clinically manifested by the alteration of cognitive processing, neurophysiologically it is displayed by an increase in cortical excitability and by a reduction of intracortical inhibition[6, 9,10,11]. On the other hand, sensitization is clinically manifested by an increase in headache parameters, neurophysiologically is displayed by an alteration of the pressure pain threshold over trigeminal and extra-trigeminal area[5, 12,13,14,15]. Taken together, lack of habituation and sensitization leads to an imbalance between excitatory-inhibitory transmission[16, 17], which in turn, results in cognitive impairment[8, 18, 19] and dysfunction in pain modulation [5, 20, 21].
Recent studies support the efficacy of non-pharmacological treatments in the management of migraine[12, 22, 23]. In the context of existing treatments for migraine, any treatments may act on sensitization pain modulation other on habituation: active exercise may result in neuroplasticity, through the release of the brain-derived neurotrophic factor, and in pain modulation, through the activation of endogenous systems[24,25,26,27,28]; cognitive training may result in neuroplasticity and global cognition, through the enhance of the performance on cognitive functions[29,30,31]. However, it seems that there are no treatments that may act both on sensitization and on habituation in people with migraine. Currently, the combination of active exercise with cognitive training in a concomitant dual task protocol represents an emerging treatment to strength their efficacy. Cognitively, a dual task protocol promotes divided attention, such as the ability to perform two or more tasks simultaneously, and executive functions, such as working memory, inhibition and shifting; physically, dual task protocol promotes gait, balance [32,33,34,35,36,37]. Moreover, it seems that the neuroplasticity induced by active exercise is further enhanced in dual task protocol, through an up-regulation of cortical connectivity[38]. These concomitant combination in a dual task protocol could act on both habituation and sensitization outcomes in migraine. To date, no previous study has investigated the efficacy of a dual task protocol in people with migraine. Furthermore, no previous study has compared the effect of different types of treatments on neurophysiological and clinical outcomes in people with migraine.
Based on previous literature [32,33,34,35,36,37], it could be hypothesized that a dual task protocol could be more effective than active exercise and cognitive training alone by combining the mechanisms of action on habituation and sensitization. Therefore, the first aim of this study was to investigate the efficacy of a dual-task protocol in people with episodic migraine with respect to both active exercises only and to cognitive training-only treatments, concerning some peripheral and central neurophysiological outcomes. The second aim was to evaluate the efficacy of a dual-task protocol in people with episodic migraine with respect to the two monotherapies concerning some clinical outcomes.
Methods
A randomized control trial was adopted in people with episodic migraine without aura (ICHD-3)[39]. The project was approved by the institutional review board (CEUR 2021-Sper-26; ID 3672) and it was registered on ClinicalTrials.gov (identifier: NCT05596058). The informed consent was written by all participants. The first evaluation and enrolment were performed by a tertiary Headache Centre. The following inclusion criteria were respected: Episodic high-frequency migraine diagnosis (ICHD-3)[39]; Age between 18 and 65 years. Exclusion criteria were: migraine with aura; contraindications or low tolerance to TMS; other neurological or psychiatric disorders; cardiac implantable devices; current drug intake that may change the cortical excitability; previous migraine prophylaxis treatment in the last three months; comorbidities such as depression, anxiety, sleep disorders; participants that do not provide their consent to the study. Each participant received a diary to record the frequency of migraine, duration of the attack, migraine intensity and drug intake per month. After one month, each patient was evaluated, and the eligibility criteria were frequency of migraine from ≥ 8 to ≤ 14 days per month, according to diagnostic criteria of ICDH3-beta [39]. The enrolment began on the first of June 2022, the primary completion was performed on the first October 2022, the data collection was completed on the first November 2023 due to the unavailability of the subjects who give the consent to participate to the study protocol.
Outcomes assessment
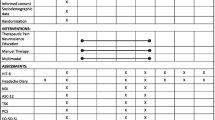
Figure 1 presents an overview of the timeline of the study (Fig. 1).
Pressure pain threshold (PPT) assessment
PPT assessment was conducted according to previous guidelines[40]. Five muscles were chosen over the trigeminocervical complex (masseter, temporalis, procerus, trapezius and sub-occipitalis), and one muscle was chosen outside the trigemino-cervical (tensor fascia latae). Participants were asked to press the stop button when the pressure was felt as painful[22, 40]. The first pressure was applied on the wrist of each participant, in order to familiarize with the procedure[22, 40]. Three evaluations were carried out for each muscle with one-minute of interval between each evaluation. A rate of approximately 30 kPa/s was increased in each assessment [12, 40].
Transcranial magnetic stimulation (TMS)
The single-pulse protocol (sp) of TMS was used to teste the resting motor threshold (rMT) while the paired pulse protocol (pp) of TMS was used to assess the short interval intracortical inhibition (SICI) and the intracortical facilitation (ICF) over the left primary motor cortex (M1)[41,42,43]. A MagPro® magnetic stimulator (MagVenture Inc., Alpharetta, GA, USA) was used by connecting it to an electromyographic device (Synergy®, Natus®, Middleton, WI, USA). The amplitude of MEPs was calculated peak-to-peak for each evaluation[21]. The TMS parameters were collected in this order: 1) From Single-Pulse (sp)-TMS, the Resting Motor Threshold (rMT): the minimum stimulation intensity required to produce a peak-to-peak motor evoked potential (MEP) amplitude of ≥ 50 μV in at least 50% of five of ten consecutive stimuli[21, 42]; 2) From Paired Pulse (pp)-TMS, short interval intracortical inhibition (SICI): evoked by delivering a subthreshold (80% rMT) conditioning stimulus followed by a suprathreshold (130% rMT) test stimulus at interstimulus intervals (ISIs) of 3 and 5 ms. Four MEPs were recorded; 3) From Paired Pulse (pp)-TMS, intracortical facilitation (ICF): evoked by delivering a subthreshold (80% rMT) conditioning stimulus followed by a suprathreshold (130% rMT) test stimulus at ISIs of 10, 15 and 20 ms. Four MEPs were recorded [21, 44].
Executive functions
The Trail Making Test (TMT) and the Frontal Assessment Battery (FAB) were used to evaluate the executive functions[8, 45, 46]. TMT is divided into TMT A and TMT B[8, 45,46,47]. FAB explores 6 functions: conceptualization, mental flexibility, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy. To reduce the risk of learning effect all participants were instructed to perform the executive functions tests at least 8 times in the week preceding the baseline evaluation.
Migraine Disability Assessment Scale (MIDAS)
The MIDAS consists of 5 questions concerning the following item [48]: absence from work or school, inability to carry out household chores, and to take part in family, social, or leisure activities.
Headache diary
From t0, the headache diary was used by participants during the 3 months of each treatment (t1) and after 1 month follow up (t2) to collect the following information: frequency of migraine; duration of attacks; pain intensity; symptomatic drugs intake; association with menstrual cycle[49]. The consumption of symptomatic medication was allowed maximum twice a week[50]. Participants that exceeded this frequency were considered dropouts in the final data analysis.
All the assessments were performed for all participants exclusively in the inter-ictal phase[51, 52]. In addition, participants had to not take any medication that may modify the cortical excitability for at least 72 h before the measurements[53]. In the case of female subjects, the evaluations were applied always in the late follicular phase [54].
After the baseline data collection (t0), participants were randomized into three groups with a stratification for age and sex (1:1:1): active exercise only, cognitive task only and dual-task protocol. One of the investigators who was not involved in outcomes assessment and physiotherapy protocols evaluated inclusion and exclusion criteria and randomized the subjects into the three groups. The examiner who performed the outcomes measure and the statistical analysis was blinded to the participants allocation. Also, participants were blinded concerning the other groups of treatments (Fig. 1).
Intervention
Active exercise-only protocol
Active exercise-only protocol was scheduled in 20 one-hour individual sessions twice per week. The protocol was based on the review of Lemmens[26]. Each training session started with a 20-min warm-up by walking on a treadmill with a progressive increase in speed every 5 min. This part was followed by 30 min of strengthening exercises for the total body. Finally, in the last 10 min stretching exercises were applied with slow breathing[26]. The circuit of the exercise was changed every two weeks, with a total of 5 graded circuits during the three months of treatment (see supplementary material).
Cognitive training only protocol
The cognitive training-only protocol was scheduled in a total of 20 one-hour individual sessions twice per week. The protocol was based on previous studies for cognitive impairment[8, 31,32,33,34,35,36] and for migraine[29]. The goal was to engage the three core executive functions: inhibition, working memory and shifting[55]. In order to increase the difficulty, the cognitive task was constantly changed. A total of 15 min was spent on the inhibition goal: GO-NO-GO tasks (5 min), Stroop tasks (5 min) and inhibition of the correct answer (5 min). During the GO-NO-GO task, participants had to provide different responses to alternating signals i.e., “not tapping when the examiner taps twice and copying the examiner when he taps once”. In the Stroop task, participants had to name the name of a color (e.g., "blue", "green", or "red") printed in a different color. In the inhibition of the correct answer task, participants were asked to give the wrong answer to simple general culture questions presented and to inhibit the impulse to answer correctly. The working memory goal lasted 15 min. Several memory tasks were presented: counting down, word list recall, spelling a five-letter word backwards, subtracting by sixes or sevens from a randomly presented two-digit number, reciting the months in the reverse order starting from a randomly chosen month. The shifting goals lasted 15 min: mental flexibility tasks (5 min), Simon tasks (5 min) and Wisconsin Card Sorting Test (5 min). In mental flexibility tasks, patients listed as many words as they could in one minute. Word categories included words beginning with a specific letter or belonging to a specific semantic category or words that were in rhyme with a word chosen by the therapist. In the Simon task a blue or red square was presented on the left or the right side of the computer screen. The participants were asked to raise their left hand if the square was blue and the right hand if the square was red, irrespectively of the location of the square. The trials were either congruent or incongruent, where in the congruent trials, the stimulus location was on the same side as the response key, and in the incongruent trials on the opposite side. In the Wisconsin Card Sorting Test, 4 cards were presented to the patients, with numbers (from 1 to 4), colors (red, green, yellow, blue) and symbols (triangle, square, circle, star). A fifth card was then shown depicting one item from each of the four categories. The patients were asked to select the card among the first 4 presented that matched the fifth card. The software randomly changed the categories after a few repetitions (see supplementary material).
Dual-task protocol
The dual-task protocol was an integrated protocol of active exercise-only protocol with concomitant cognitive training-only protocol. A total of 20 one-hour individual sessions were conducted twice per week. Participants had to perform different cognitive tasks during concomitant active exercise scheduled in 5 graded circuits[32] (see supplementary material).
After 3 months of each treatment, participants were revaluated (t1) with the same neurophysiological and clinical outcomes (PPT; TMS; TMT; FAB; MIDAS; headache diary) and after 1-month follow-up with the same outcomes (PPT; TMS; TMT; FAB; MIDAS; headache diary) (Fig. 1).
Statistical analyses
This is the primary analysis of this data. G*Power was used to demine posteriori the achieve power considering the main finding of this study, being > 0.95. The statistical analyses were performed with SPSS version 23 (IBM). Data are reported as means, standard deviations (SDs), and 95% confidence intervals (CI), or counts and proportions (%) as appropriate. Two-tailed testing was performed. To evaluate the effect of the different treatment protocols on the assessed outcomes, a mixed-factors analysis of variance (ANOVA) was performed for the different measures (within-groups: time – t0, t1, t2; between-groups: treatment—dual tasks, active exercise, cognitive training), considering also the possible effect of interstimulus interval for TMS (SICI, within-groups: ISI—3 ms, 5 ms; ICF, within-groups: ISI—10 ms, 15 ms, 20 ms), and the possible effect of the side of the body for PPT (within-groups: side—right, left). In case of significant main effects, a postdoc analysis with Sidak's correction was performed. Due to the large variability within groups for SICI at 3 ms, a repeated measures ANOVA was also performed separately for each group. Normality testing was performed by using the Shapiro–Wilk test was on all the datasets. Significance was set at p < 0.05.
Results
A total of 30 adults with episodic high-frequency migraine were included (Fig. 2)[56]. The active exercise-only group consisted of 2 men and 8 females (36.5 ± 13.9); the cognitive task-only group consisted of 2 men and 8 females (mean age 42.7 ± 11.2); the dual task protocol group consisted of 2 men and 8 females (mean age 48.3 ± 9.7). There was no missing data.
Pressure Pain Threshold
No time x group x side, time x side, nor group x side effects were observed for any of the assessed muscles. A significant time x group effect was only found for the trapezius muscle (F4,54 = 3.588, p = 0.012, pη2 = 0.210). A significant time effect was found for the procerus muscle (F2,54 = 3.472, p = 0.038, pη2 = 0.114), but not for the other assessed muscles. In addition, a significant side effect was only found for the trapezius muscle (F1,27 = 12.719, p = 0.001, pη2 = 0.320). In particular, the PPT over the trapezius muscle was suggested to increase over time in the dual task group and in the active exercises group, whereas in the cognitive training group the PPT decreased over time (Table 1). Regarding the procerus muscle, the pain threshold did not significantly increase between t0 and t1 (28.2 kPa; 95% CI: -15.1—71.4; p = 0.293), between t1 and t2 (17.6 kPa; 95% CI: -12.1—47.3.5; p = 0.369), and between t0 and t2 (45.7 kPa; 95% CI: -11.0—102.5; p = 0.142).
Transcranial Magnetic Stimulation (TMS)
Single-pulse TMS Protocol
No time x group effect (F4,54 = 0.501, p = 0.735, pη2 = 0.036) and no group effect (F2,27 = 0.443, p = 0.647, pη2 = 0.032) were found for rMT, while a statistically significant time effect was observed (F2,54 = 24.208, p < 0.001, pη2 = 0.473) (Table 2). In fact, it significantly increased from t0 to t1 (13.8%; 95% CI: 7.1–20.5; p < 0.001), and between t0 and t2 (15.6%; 95% CI: 8.2–23.1; p < 0.001), but not from t1 to t2 (1.8%; 95% CI: -2.3—6.0; p = 0.608).
Paired-pulse TMS protocol
Concerning SICI, only a significant ISI effect was found (F1,27 = 6.896, p = 0.014, pη2 = 0.203). In fact, the MEP value was significantly higher at 5 ms than 3 ms (0.171 mV; 95% CI: 0.037–0.304; p = 0.014). A repeated measures.
ANOVA for SICI at 3 ms highlighted a statistically significant time effect for the dual task group (F2,18 = 15.251,
p < 0.001, pη2 = 0.629), but not for the active exercises group (F2,18 = 0.589, p = 0.565, pη2 = 0.061), and for the cognitive training (F2,18 = 1.090, p = 0.357, pη2 = 0.108). In fact, for the dual task group the MEP value at 3 ms significantly decreased between t0 and t1 (-0.149 mV; 95% CI: -0.230—-0.068; p = 0.001), and from t0 to t2 (0.138 mV; 95% CI: -0.247—-0.029; p = 0.015), but not between t1 and t2 (0.011 mV; 95% CI: -0.058—0.080 p = 0.956). In the active exercises group, the MEP value at 3 ms did not significantly decrease from t0 to t1 (-0.001 mV; 95% CI: -0.582—0.580; p > 0.999), between t0 and t2 (-0.150 mV; 95% CI: -0.509—0.209; p = 0.583), and from t1 to t2 (-0.149 mV; 95% CI: -0.576—0.278; p = 0.705). In the cognitive training group, the MEP value at 3 ms significantly decreased between t0 and t2 (-0.079 mV; 95% CI: -0.151—-0.008; p = 0.030), but not from t0 to t1 (-0.081 mV; 95% CI: -0.307—0.470; p = 0.913), and not between t1 and t2 (-0.160 mV; 95% CI: -0.543—0.222; p = 0.581) (Fig. 3). Concerning ICF, only a significant time x ISI effect was observed (F4,108 = 2.909, p = 0.048, pη2 = 0.097) (Table 2).
Transcranial Magnetic Stimulation pared pulse protocol. Transcranial magnetic stimulation (TMS) motor evoked potentials (MEPs) at different interstimulus intervals (ISI) of participants with migraine (n=10) before the dual task protocol (t0) after 3 months of dual task protocol (t1) and after 1-month follow-up (t2)
Cognitive functions
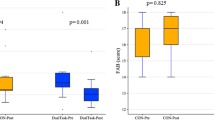
A significant time effect was observed for TMT (F2,54 = 13.812, p < 0.001, pη2 = 0.338), which significantly improved between t0 and t1 (-6.2 s, 95% CI: -10.5—-1.8, p = 0.004), and from t0 to t2 (-9.2 s, 95% CI: -14.1—-4.3, p < 0.001), whereas it was not statistically significant between t1 and t2 (- 3.1 s, 95% CI: -7.5 –1.3, p = 0.238). A significant time effect was found in the FAB assessment (F2,54 = 23.173, p < 0.001, pη2 = 0.462), showing a significant improvement between t0 and t1 (1.1, 95% CI: 0.5 – 1.8, p < 0.001), and from t0 to t2 (1.3, 95% CI: 0.7 – 1.8, p < 0.001), whereas it was not statistically significant between t1 and t2 (0.1, 95% CI: -0.2 –0.5, p = 0.707) (Table 3).
MIDAS
No time x group effect (F2,27 = 0.264, p = 0,770, pη2 = 0.019), nor no group effect were observed (F2,27 = 0.255, p = 0.776, pη2 = 0.019), while a statistically significant time effect was found (F1,27 = 20.006, p < 0.001, pη2 = 0.426). Indeed, the MIDAS scores significantly decreased from t0 to t1 (-26.9; 95% CI: -39.2—-14.6; p < 0.001) (Table 4).
Headache diary
No significant time x group nor group effects were observed for the assessed parameters. No time effects were found about low-intensity pain (F2,54 = 0.018, p = 0.982, pη2 = 0.001) and medium-intensity pain (F2,54 = 1.664, p = 0.199, pη2 = 0.058), while a statistically significant effect was observed for frequency (F2,54 = 6.461, p = 0.003, pη2 = 0.193), duration of attack (F2,54 = 4.443, p = 0.016, pη2 = 0.141), high-intensity pain (F2,54 = 3.738, p = 0.030, pη2 = 0.122), and drugs intake (F2,54 = 8.652, p = 0.001, pη2 = 0.243). Frequency significantly decreased from t0 to t2 (-2.6; 95% CI: -4.4—-0.8; p = 0.003), but not between t0 and t1 (-1.8; 95% CI: -4.0—0.4; p = 0.142), and not from t1 to t2 (-0.8; 95% CI: -2.5 – 0.8; p = 0.488). Drugs intake significantly decreased between t0 and t1 (-3.9; 95% CI: -6.8—-1.0; p = 0.007), and from t0 to t2 (-4.0; 95% CI: -7.3—-0.7; p = 0.014), but not between t1 and t2 (-0.1; 95% CI: -2.0—1.9; p > 0.999) (Table 4).
Discussion
The present study has evaluated, for the first time, the efficacy of a dual task protocol, i.e., active exercise plus cognitive task, on neurophysiological and clinical outcomes related to sensitization and habituation in people with migraine. The first interesting finding was that the pressure pain threshold (PPT) increased only in active exercise and dual-task protocol groups, suggesting a more positive effect on sensitization. On the other side, resting motor threshold, migraine-related disability, and neurophysiological tests improved, without differences, among all groups. Finally, the most remarkable finding was that short intracortical inhibition seems to be normalized only by the dual task protocol, suggesting a more effective action on the lack of habituation. Furthermore, the dual task protocol reported a higher migraine responders’ rate, suggesting a more effective action also on sensitization.
With regard to PPT, the findings supported the idea that active exercise could have a desensitization effect. Although no differences were reported between the active exercise only and dual-task group, the dual task seems to be more useful: the dual task protocol increased the PPT over 11 out of 11 muscles at both t1 and t2; the active exercise increased the PPT over 8 out of 11 muscles at t1, and over 9 out of 11 at t2. On the other side, the cognitive training only seems not to change the PPT: in fact, the PPT increased over only 3 out of 11 muscles at both t1 and over 1 of 11 at t2. No previous study had investigated the PPT variation after a dual-task protocol. Even though, previous research on people with chronic migraine showed a reduction of the PPT over all the muscles after an integrated protocol of active exercise and manual therapy[12]. This result supported the peripheral and central desensitization action of active exercise[24, 25, 28]: peripherally through the suppression of pain signals in the spinal dorsal horn, centrally through the activation of the brain areas involved in pain modulation. Particularly for migraine, it seems that high-intensity exercise has a direct effect on calcitonin gene-related peptide (CGRP): the release of the endocannabinoid ligand anandamide (AEA) inhibits the vasodilatation of the dural induced by CGRP[57]. This, in turn, may lead to a reduction of the PPT. It can be suggested, therefore, that dual-task protocol with active exercise may act on sensitization in people with migraine.
Concerning rMT, it reflects the excitability of cortico-cortical axons[58]. The resting motor threshold (rMT) increased after the all the three treatments, without differences. This result supports that active exercise increases motor evocated potential (MEP) due to a neuroplasticity mechanism [59,60,61,62]. No previous research highlighted change in rMT after cognitive training, but previous research has highlighted that cognitive training can promote neuroplasticity and global cognition in cognitive impairment[29, 31, 32]. The increase of MEP in people with migraine after cognitive training may be related to the rise in the performance of executive functions, which, in turn, leads to an improved lack of habituation. Consequently, the association of active exercise with concomitant cognitive tasks in a dual-task protocol may be more effective on the neuroplasticity mechanism in the motor cortex. Despite the analysis of variance did not report significant interaction among groups, rMT was enhanced more by three months of the dual task protocol with respect to the two monotherapies. Therefore, it is possible to hypothesize that the dual task may reduce cortical excitability due to a lack of habituation.
SICI and ICF represent a neurophysiological outcome of the inhibitory circuits mediated by GABAergic neurotransmission (SICI) and excitatory mediated by glutamatergic neurotransmission (ICF), respectively [63]. Studies agree on a significant reduction of SICI in people with migraine, but the data regarding ICF are still not clear [11, 64,65,66,67]. Our finding, for the first time, suggests that the dual task protocol may normalize the inhibitory circuits mediated by the GABAergic neurotransmission (SICI). This normalization was not highlighted in the two monotherapies. In fact, the neuroplasticity induced by the two monotherapies seems to be strongly increased by their concomitant association: a dual-task protocol may up-regulate the cortical connectivity related to cognitive tasks through activity-dependent learning that strengthens the synapses associated with the cognitive tasks [38]. Therefore, this up-regulation may promote associative learning and neuroplasticity and, consequently, may reduce the lack of habituation.
As regards cognitive functions, all treatments improve cognitive performance without differences among groups. It seems that cognitive impairment in people with migraine is due to central sensitization, migraine comorbidity/disability and lack of habituation. Active exercise may act centrally, with the release of the brain-derived neurotrophic factor that promotes cognitive performance and neuroplasticity, and peripherally, with the reduction of central sensitization that restores cognitive functioning [24, 25, 28]. On the other hand, cognitive training directly enhances executive functions that may reduce migraine-related disability and lack of habituation. Finally, the cognitive performance obtained after the dual-task protocol may reflect an action of the lack of habituation: on the one side the divided attention is promoted by the performance of two or more tasks simultaneously, on the other side, associative learning is potentiated by the up-regulation of synapses related to the cognitive tasks[38].
Concerning MIDAS and headache parameters, no differences were highlighted among the three treatments. However, the dual task protocol seems to have a more evident effect with respect to the two monotherapies in migraine-related disability, duration of migraine, drug intake. Data suggest also a less evident reduction after cognitive training only of all headache parameters. In line with the literature, data confirm that active exercise may be more effective in the reduction of migraine frequency in respect to the reduction of pain intensity[26, 68]. It is possible that the dual-task protocol is more effective in the improvement of clinical parameters with respect to active exercise only and cognitive training only.
The present study presents some limitations; first of all, the small sample size could have impacted statistical power and therefore suggests caution when interpreting the statistical findings; in addition, it did not allow sex stratification. In fact, sex plays a role in pain modulation and cortical excitability. In order to limit this variable, the proportion of males and females was the same in all the tested groups. Second, the guidelines of the International Headache Society allow the consumption of symptomatic medication [50]. Therefore, the only possibility was to limit the symptomatic drug intake to twice a week and to exclude the final analysis of the participants that exceeded this limit, as in previous studies on this topic [50]. Third, a long-term follow up would be necessary to determine the sustainability of the interventions’effects over time. Third, a long-term follow up would be necessary to determine the sustainability of the interventions’ effects over time. Despite these limitations, the study presents some strong novel points: first, the efficacy of an emerging non-pharmacological treatment, i.e., dual-task protocol, was investigated for the first time in people with migraine; second, the neurophysiological effects of three non-pharmacological treatments were assessed for the first time in people with migraine; third, the clinical effects of three non-pharmacological treatments were compared for the first time in people with migraine.
Conclusion
A dual task protocol, with concomitant cognitive training and active exercise, in people with migraine seems to be useful both in habituation and in sensitization outcomes. To evaluate the generalizability of our findings, future studies are encouraged on larger samples, including sex and age stratification. In addition, long-term follow up could assess the sustainability of the intervention’s effects over time and allow a deeper investigation of the differences among treatments. Finally, the effect of the combination of both pharmacological treatments and dual task protocols on habituation and sensitization outcomes is still to be investigated and could provide an added value for clinical translation of these findings. Since different migraine populations and migraine types might respond differently to the proposed interventions, future studies are encouraged to evaluate the effects of the dual task protocol in different migraine types, such as chronic migraine and migraine with aura. Finally, these potential mechanisms observed in the dual-task protocol should be investigated in other pathologies and also with other method such as blink reflex, Contingent negative variation and P300, auditory, visual and somatosensory evoked potential, Blood flow and oxygen consumption with functional neuroimaging [4].
Data Availability
The principal author takes full responsibility for the data presented in this study, analysis of the data, conclusions, and conduct of the research. The datasets page containing authors’ details analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PPT:
-
Pressure pain threshold
- TMS:
-
Transcranial magnetic stimulation
- rMT:
-
Resting motor threshold
- ISIs:
-
Interstimulus intervals
- Sp TMS:
-
Single-pulse transcranial magnetic stimulation
- PP TMS:
-
Paired pulse transcranial magnetic stimulation
- SICI:
-
Short interval intracortical inhibition
- ICF:
-
Intracortical facilitation
- FAB:
-
Frontal assessment Battery
- TMT:
-
Trail making test
- MIDAS:
-
Migraine disability assessment scale
- CGRP:
-
Calcitonin gene-related peptide
- MEP:
-
Motor evocated potential
References
Agosti R (2018) Migraine Burden of Disease: From the Patient’s Experience to a Socio-Economic View. Headache 58:. https://doi.org/10.1111/head.13301
Abbafati C, Abbas KM, Abbasi-Kangevari M, et al (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:. https://doi.org/10.1016/S0140-6736(20)30925-9
Goadsby PJ, Holland PR, Martins-Oliveira M, et al (2017) Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev 97:. https://doi.org/10.1152/physrev.00034.2015
Coppola G, Pierelli F, Schoenen J (2009) Habituation and migraine. Neurobiol Learn Mem 92:. https://doi.org/10.1016/j.nlm.2008.07.006
Coppola G, di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14. https://doi.org/10.1186/1129-2377-14-65
de Tommaso M, Ambrosini A, Brighina F et al (2014) Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 10. https://doi.org/10.1038/nrneurol.2014.14
Dodick DW (2018) A Phase-by-Phase Review of Migraine Pathophysiology. Headache 58:. https://doi.org/10.1111/head.13300
Vuralli D, Ayata C, Bolay H (2018) Cognitive dysfunction and migraine. J Headache Pain 19:. https://doi.org/10.1186/s10194-018-0933-4
Brigo F, Storti M, Nardone R, et al (2012) Transcranial magnetic stimulation of visual cortex in migraine patients: A systematic review with meta-analysis. J Headache Pain 13. https://doi.org/10.1007/s10194-012-0445-6
Coppola G, di Renzo A, Tinelli E, et al (2016) Thalamo-cortical network activity during spontaneous migraine attacks. Neurology 87:. https://doi.org/10.1212/WNL.0000000000003327
Coppola G, di Lenola D, Abagnale C, et al (2020) Short-latency afferent inhibition and somato-sensory evoked potentials during the migraine cycle: Surrogate markers of a cycling cholinergic thalamo-cortical drive? J Headache Pain 21:. https://doi.org/10.1186/s10194-020-01104-7
Deodato M, Granato A, Ceschin M, et al (2022) Algometer Assessment of Pressure Pain Threshold After Onabotulinumtoxin-A and Physical Therapy Treatments in Patients With Chronic Migraine: An Observational Study. Front Pain Res 3:. https://doi.org/10.3389/fpain.2022.770397
di Antonio S, Castaldo M, Ponzano M, et al (2022) Trigeminal and cervical sensitization during the four phases of the migraine cycle in patients with episodic migraine. Headache 62:. https://doi.org/10.1111/head.14261
Deodato M, Granato A, Martini M et al (2024) Instrumental assessment of pressure pain threshold over trigeminal and extra-trigeminal area in people with episodic and chronic migraine: a cross-sectional observational study. Neurol Sci. https://doi.org/10.1007/s10072-024-07372-4
Deodato M, Granato A, Del Frate J, et al (2024) Differences in musculoskeletal dysfunctions and in postural alterations between chronic migraine and chronic tension type headache: A cross-sectional study. J Bodyw Mov Ther 37. https://doi.org/10.1016/j.jbmt.2023.11.011
Bathel A, Schweizer L, Stude P, et al (2018) Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain 19:. https://doi.org/10.1007/10194.1129-2377
Peek AL, Leaver AM, Foster S, et al (2021) Increased GABA+ in People With Migraine, Headache, and Pain Conditions- A Potential Marker of Pain. J Pain 22:. https://doi.org/10.1016/j.jpain.2021.06.005
Rist PM, Kurth T (2013) Migraine and cognitive decline: A topical review. Headache 53. https://doi.org/10.1111/head.12046
Schmid S, Wilson DA, Rankin CH (2015) Habituation mechanisms and their importance for cognitive function. Front Integr Neurosci 8. https://doi.org/10.3389/fnint.2014.00097
Charles A (2018) The pathophysiology of migraine: implications for clinical management. Lancet Neurol 17. https://doi.org/10.1016/S1474-4422(17)30435-0
Deodato M, Granato A, Martini M et al (2023) Neurophysiological and Clinical Outcomes in Episodic Migraine Without Aura: A Cross-Sectional Study. J Clin Neurophysiol. https://doi.org/10.1097/WNP.0000000000001055
Deodato M, Granato A, Borgino C, et al (2022) Instrumental assessment of physiotherapy and onabolulinumtoxin-A on cervical and headache parameters in chronic migraine. Neurol Sci 43:. https://doi.org/10.1007/s10072-021-05491-w
Pourahmadi M, Dommerholt J, Fernández-De-Las-Peñas C, et al (2021) Dry Needling for the Treatment of Tension-Type, Cervicogenic, or Migraine Headaches: A Systematic Review and Meta-Analysis. Phys Ther 101. https://doi.org/10.1093/ptj/pzab068
Amin FM, Aristeidou S, Baraldi C, et al (2018) The association between migraine and physical exercise. J Headache Pain 19. https://doi.org/10.1186/s10194-018-0902-y
da Silva Santos R, Galdino G (2018) Endogenous systems involved in exercise-induced analgesia. J Physiol Pharmacol 69. https://doi.org/10.26402/jpp.2018.1.01
Lemmens J, de Pauw J, van Soom T, et al (2019) The effect of aerobic exercise on the number of migraine days, duration and pain intensity in migraine: A systematic literature review and meta-analysis. J Headache Pain 20. https://doi.org/10.1186/s10194-019-0961-8
Barber M, Pace A (2020) Exercise and Migraine Prevention: a Review of the Literature. Curr Pain Headache Rep 24. https://doi.org/10.1007/s11916-020-00868-6
Song TJ, Chu MK (2021) Exercise in Treatment of Migraine Including Chronic Migraine. Curr Pain Headache Rep 25. https://doi.org/10.1007/s11916-020-00929-w
Precenzano F, Ruberto M, Parisi L, et al (2017) Visual–spatial training efficacy in children affected by migraine without aura: A multicenter study. Neuropsychiatr Dis Treat 13:. https://doi.org/10.2147/NDT.S119648
Nguyen L, Murphy K, Andrews G (2019) Cognitive and neural plasticity in old age: A systematic review of evidence from executive functions cognitive training. Ageing Res Rev 53. https://doi.org/10.1016/j.arr.2019.100912
Brissart H, Omorou AY, Forthoffer N, et al (2020) Memory improvement in multiple sclerosis after an extensive cognitive rehabilitation program in groups with a multicenter double-blind randomized trial. Clin Rehabil 34:. https://doi.org/10.1177/0269215520920333
Deodato M, Qualizza C, Martini M et al (2024) Efficacy of dual-task augmented reality rehabilitation in non-hospitalized adults with self-reported long COVID fatigue and cognitive impairment: a pilot study. Neurol Sci. https://doi.org/10.1007/s10072-023-07268-9
Kim E, Yun SJ, Oh BM, Seo HG (2023) Changes of neural coupling between cognitive and motor networks associated with dual-task performance in Parkinson’s disease. Neurol Sci. https://doi.org/10.1007/s10072-023-07255-0
Onder H, Ozyurek O (2021) The impact of distinct cognitive dual-tasks on gait in Parkinson’s disease and the associations with the clinical features of Parkinson’s disease. Neurol Sci 42:. https://doi.org/10.1007/s10072-020-04874-9
Sütçü G, Doğan M, Topuz S (2022) Investigation of postural control and spatiotemporal parameters of gait during dual tasks in ataxic individuals. Neurol Sci. https://doi.org/10.1007/s10072-022-06248-9
Zhang M, Gan Y, Wang X, et al (2023) Gait performance and non-motor symptoms burden during dual-task condition in Parkinson’s disease. Neurol Sci 44:. https://doi.org/10.1007/s10072-022-06411-2
Du YH, Ma J, Hu JY, et al (2023) Effects of dual-task training on gait and motor ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 37:. https://doi.org/10.1177/02692155221146085
Perini R, Bortoletto M, Capogrosso M, et al (2016) Acute effects of aerobic exercise promote learning. Sci Rep 6:. https://doi.org/10.1038/srep25440
Olesen J (2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38. https://doi.org/10.1177/0333102417738202
Andersen S, Petersen MW, Svendsen AS, Gazerani P (2015) Pressure pain thresholds assessed over temporalis, masseter, and frontalis muscles in healthy individuals, patients with tension-type headache, and those with migraine-a systematic review. Pain 156. https://doi.org/10.1097/j.pain.0000000000000219
Cosentino G, di Marco S, Ferlisi S, et al (2018) Intracortical facilitation within the migraine motor cortex depends on the stimulation intensity. A paired-pulse TMS study. J Headache Pain 19:. https://doi.org/10.1186/s10194-018-0897-4
Rossini PM, Burke D, Chen R, et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application: An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126. https://doi.org/10.1016/j.clinph.2015.02.001
Rossi S, Antal A, Bestmann S, et al (2021) Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin Neurophysiol 132. https://doi.org/10.1016/j.clinph.2020.10.003
Manganotti P, Michelutti M, Furlanis G, et al (2023) Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clinical Neurophysiology 151:. https://doi.org/10.1016/j.clinph.2023.04.010
le Pira F, Reggio E, Quattrocchi G, et al (2014) Executive dysfunctions in migraine with and without aura: What is the role of white matter lesions? Headache 54:. https://doi.org/10.1111/head.12158
Vallesi A (2020) On the utility of the trail making test in migraine with and without aura: A meta-analysis. J Headache Pain 21. https://doi.org/10.1186/s10194-020-01137-y
Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S et al (2017) The Trail Making Test: Association With Other Neuropsychological Measures and Normative Values for Adults Aged 55 Years and Older From a Spanish-Speaking Population-Based Sample. Assessment 24:183–196. https://doi.org/10.1177/1073191115602552
Peng KP, Wang SJ (2012) Migraine diagnosis: Screening items, instruments, and scales. Acta Anaesthesiologica Taiwanica 50. https://doi.org/10.1016/j.aat.2012.05.002
Nappi G, Jensen R, Nappi RE, et al (2006) Diaries and calendars for migraine. A review. Cephalalgia 26. https://doi.org/10.1111/j.1468-2982.2006.01155.x
Tassorelli C, Diener HC, Dodick DW, et al (2018) Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 38:. https://doi.org/10.1177/0333102418758283
Toriyama T, Horiuchi T, Hongo K (2017) Characterization of migraineurs presenting interictal widespread pressure hyperalgesia identified using a tender point count: a cross-sectional study. J Headache Pain 18:. https://doi.org/10.1186/s10194-017-0824-0
Peng KP, May A (2018) Quantitative sensory testing in migraine patients must be phase-specific. Pain 159. https://doi.org/10.1097/j.pain.0000000000001353
Ziemann U, Reis J, Schwenkreis P, et al (2015) TMS and drugs revisited 2014. Clin Neurophysiol 126. https://doi.org/10.1016/j.clinph.2014.08.028
Delaruelle Z, Ivanova TA, Khan S, et al (2018) Male and female sex hormones in primary headaches. J Headache Pain 19. https://doi.org/10.1186/s10194-018-0922-7
Diamond A (2013) Executive functions. Annu Rev Psychol 64. https://doi.org/10.1146/annurev-psych-113011-143750
Schulz KF, Altman DC, Moher D (2010) CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Italian J Public Health 7:. https://doi.org/10.4178/epih/e2014029
Akerman S, Kaube H, Goadsby PJ (2004) Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol 142:. https://doi.org/10.1038/sj.bjp.0705896
Badawy RAB, Loetscher T, Macdonell RAL, Brodtmann A (2012) Cortical excitability and neurology: Insights into the pathophysiology. Funct Neurol 27. PMC3812767
Singh AM, Staines WR (2015) The effects of acute aerobic exercise on the primary motor cortex. J Mot Behav 47. https://doi.org/10.1080/00222895.2014.983450
Yamazaki Y, Sato D, Yamashiro K, et al (2019) Acute Low-Intensity Aerobic Exercise Modulates Intracortical Inhibitory and Excitatory Circuits in an Exercised and a Non-exercised Muscle in the Primary Motor Cortex. Front Physiol 10:. https://doi.org/10.3389/fphys.2019.01361
Mellow ML, Goldsworthy MR, Coussens S, Smith AE (2020) Acute aerobic exercise and neuroplasticity of the motor cortex: A systematic review. J Sci Med Sport 23. https://doi.org/10.1016/j.jsams.2019.10.015
Morris TP, Fried PJ, Macone J, et al (2020) Light aerobic exercise modulates executive function and cortical excitability. Eur J Neurosci 51:. https://doi.org/10.1111/ejn.14593
Lefaucheur JP (2019) Transcranial magnetic stimulation. In: Handbook of Clinical Neurology 160. https://doi.org/10.1016/B978-0-444-64032-1.00037-0
Aurora SK, Barrodale P, Chronicle EP, Mulleners WM (2005) Cortical inhibition is reduced in chronic and episodic migraine and demonstrates a spectrum of illness. Headache 45:. https://doi.org/10.1111/j.1526-4610.2005.05108.x
Siniatchkin M, Kröner-Herwig B, Kocabiyik E, Rothenberger A (2007) Intracortical inhibition and facilitation in migraine - A transcranial magnetic stimulation study. Headache 47:. https://doi.org/10.1111/j.1526-4610.2007.00727.x
Neverdahl JP, Omland PM, Uglem M, et al (2017) Reduced motor cortical inhibition in migraine: A blinded transcranial magnetic stimulation study. Clinical Neurophysiology 128:. https://doi.org/10.1016/j.clinph.2017.08.032
Alaydin HC, Vuralli D, Keceli Y, et al (2019) Reduced Short-Latency Afferent Inhibition Indicates Impaired Sensorimotor Integrity During Migraine Attacks. Headache 59:. https://doi.org/10.1111/head.13554
Irby MB, Bond DS, Lipton RB, et al (2016) Aerobic Exercise for Reducing Migraine Burden: Mechanisms, Markers, and Models of Change Processes. Headache 56. https://doi.org/10.1111/head.12738
Acknowledgements
The authors are thankful to the patients for their participation. The authors want to acknowledge all the participants.
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. The study did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: Manuela Deodato, Antonio Granato, Miriam Martini, Alex Buiote Stella, Luigi Murena and Paolo Manganotti. Analysis and interpretation of data: Manuela Deodato, Alex Buiote Stella, Miriam Martini, Alessandra Galmonte, Enrico Marchetti and Ilaria Lise. Investigation: Manuela Deodato, Miriam Martini, Ilaria Lise, Enrico Marchetti. Methodology: Manuela Deodato, Antonio Granato, Miriam Martini, Luigi Murena, Alessandra Galmonte and Paolo Manganotti; Project administration: Manuela Deodato, Antonio Granato, Alex Buiote Stella and Paolo Manganotti. Resources: Manuela Deodato, Antonio Granato, Luigi Murena and Paolo Manganotti. Software: Alex Buiote Stella, Manuela Deodato, Miriam Martini, Enrico Marchetti, Ilaria Lise. Supervision: Luigi Murena, Paolo Manganotti. Validation: Manuela Deodato, Antonio Granato, Alex Buiote Stella, Luigi Murena and Paolo Manganotti. Visualization: Manuela Deodato, Antonio Granato, Alex Buiote Stella, Enrico Marchetti, Ilaria Lise and Paolo Manganotti. Writing original draft: Manuela Deodato, Antonio Granato, Alex Buiote Stella, Miriam Martini, Alessandra Galmonte, Enrico Marchetti, Ilaria Lise. Revising it critically for important intellectual content: Manuela Deodato, Paolo Manganotti, Alex Buoite Stella, Luigi Murena and Antonio Granato. Final approval of the version to be published: Manuela Deodato, Antonio Granato, Miriam Martini, Enrico Marchetti, Ilaria Lise, Alex Buiote Stella, Alessandra Galmonte, Luigi Murena, Paolo Manganotti. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional review board (CEUR 2021-Sper-26; ID 3672) and it was registered on ClinicalTrials.gov (identifier: NCT05596058).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data.
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deodato, M., Granato, A., Buoite Stella, A. et al. Efficacy of a dual task protocol on neurophysiological and clinical outcomes in migraine: a randomized control trial. Neurol Sci 45, 4015–4026 (2024). https://doi.org/10.1007/s10072-024-07611-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-024-07611-8