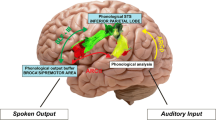
Abstract
Introduction
Learning is a long-term memory process heavily influenced by the control processes implemented by working memory, including recognition of semantic properties of items by which subjects generate a semantic structure of engrams.
Aim
The aim of this study is to investigate the verbal learning strategies of patients affected by a tumor in the left frontal lobe to highlight the role of area 9.
Method
Ten patients with frontal low-grade gliomas and ten healthy control subjects, matched for age, sex and education, were recruited and then evaluated with a two-part verbal learning test: multi-trial word list learning in free recall, and multi-trial word list learning preceded by an explicit semantic strategy cue. Frontal patients were divided into two groups: those either with frontal lesions involving or sparing area 9.
Results
In comparison to healthy control subjects, frontal patients with lesions involving area 9 memorized fewer words and displayed difficulty in using semantic strategies. When the strategy was suggested by the examiner, their performance improved, but to a lesser extent than the healthy control. Conversely, frontal patients with lesions sparing area 9 showed similar results to healthy control subjects.
Conclusion
The results suggested that, while the identification of the categorical criterion requires the integrity of the entire dorsolateral prefrontal area, only area 9, and not the surrounding areas, could be responsible for the effective use of semantic strategies in learning tasks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Learning is a complex function that involves both short and long-term memory. It is achieved through typically mnesic processes (encoding, storage, recall) but also through regulatory processes including the implementation of learning strategies.
When a verbal learning test is administered to normal subjects, they tend to implement clustering strategies [1, 2] to improve items recall. If the learning material consists of a number of words pertaining to different semantic categories, the amount of recalled words correlates with subjects’ inclination to group those words by semantic category [3, 4].
Prefrontal lesions seem to impair the ability to figure out criteria for effectively organizing the items that have to be encoded, undermining the strategical retrieval of engrams from the long-term memory store [5,6,7]. Even though frontal patients struggle to spontaneously rely on semantic organization criteria, their performance in word list learning tasks [6,7,8,9] benefits from semantic cues when explicitly suggested [6, 8, 10]. This could mean that frontal patients are generally impaired in figuring out an appropriate learning strategy, and that the strategic cue may compensate for this deficit.
In less recent literature, distinctions between lesion sites in the frontal cortex were either not carried out [6, 9, 11,12,13] or, whenever applied, they were based on the damaged hemisphere (right side vs left side) [14,15,16]. The association between frontal injury sites and patterns of impairment began to be explored further from the 1990s. A fundamental contribution came from an early study by Stuss and colleagues [7], in which the authors subdivided frontal patients into subgroups based on lesion site. Stuss and colleagues demonstrated the association between damage in different frontal areas and patterns of impairment, suggesting the involvement of distinct neural mechanisms. Addressing the discrepancies among various studies and their corresponding interpretations of learning impairment in frontal lobe damage, Alexander and colleagues [17] emphasized that there is no single, homogeneous frontal lobe syndrome in relation to memory impairment. The authors demonstrated that different subgroups of frontal patients showed different performances in the California Verbal Learning Test. They also underlined that, out of all these subgroups, only patients with lesions in the left posterior dorsolateral frontal region or in the posterior medial one exhibited an impairment in learning processes. On the basis of the interpretation of Alexander and colleagues, it is important to highlight that previous discordances between studies on verbal learning in frontal patients may originate from the lesion site being a somewhat confounding factor: the most represented lesion site may determine the main features of memory impairment, leading the authors to assign a specific deficit (and function) to the whole dorso-lateral prefrontal cortex. On the other hand, effects of a lesion in a critical site might be mitigated by a certain number of frontal patients with lesions in non-critical sites.
Dorsolateral prefrontal cortex
While item retention in short-term memory (STM) is related to the temporal [18] and parietal lobes [19], concurrent item manipulation (working memory, WM) correlates better with PFC activity.
According to Petrides [20,21,22,23], frontal lobes exchange information with posterior regions through bi-directional connections between the posterior cortical associative areas and the ventrolateral PFC (VLPFC), connected to the mid-dorsolateral PFC (DLPFC). From this perspective, VLPFC acts as the middle station that mediates the interconnection between posterior areas and DLPFC, thus playing an important role in second-level memory processes, such as the intentional retrieval of information from the long-term memory store. Instead, DLPFC is fundamental in higher memory functions such as active item manipulation and monitoring of already learnt information, as underlined by fMRI studies [24].
The use of reorganization strategies, carried out by the DLPFC, is based on the recognition of specific properties of items and enhances learning by strengthening the association between items in the long-term memory store [25]. Hence, DLPFC should be considered as a neural system not only able to encode new memories, but also able to contribute to the retrieval of old ones [26], improving engram consolidation and learning.
We refer to the WM component which selects and elaborates specific properties of items as “strategic memory”.
Alexander and colleagues [5] evaluated that critical lesions—in terms of a stronger association with impairments in learning and strategy implementation (i.e., a failure to use semantic organization)—were mainly located in the left superior frontal lobe (which they referred to as “area 9 s”). They proposed that left superior lesions may compromise the process of strategy implementation, supporting this hypothesis with the evidence [27] that temporary alterations in area 9’s activity, by means of repetitive transcranial magnetic stimulation, impaired the organization of verbal encoding.
Issues in defining a specific cognitive role of Brodmann’s area 9 (BA9), as a component of the PFC, mostly come from the adoption of different classification systems for cortical areas, and the variety of experimental protocols utilized over decades of research.
At first, Brodmann outlined his well-known classification of brain areas, in which he located area 9 in both the superior frontal gyrus and the middle frontal gyrus and, for the most part, subsequent studies on frontal functions were based on this formulation. However, addressing discrepancies between Brodmann’s classification of human brain areas and Walker’s corresponding classification for macaque monkeys [28], Petrides and Pandya [29] were able to develop a modern classification for BA9. The authors suggested a further breakdown of the original BA9 based on cytoarchitectonic properties in area 9/46 (in the middle frontal gyrus) and area 9 (in the superior frontal gyrus). Here, area 9 is restricted to the dorsomesial component of the anterior frontal cortex, laying on the superior frontal gyrus and extending medially to the paracingulate sulcus [29]. For the sake of clarity, the term “area 9 (A9)” will henceforth indicate the region described in Petrides and Pandya’s studies, therefore excluding area 9/46.
The aim of our study is to demonstrate that implementation of semantic clustering strategies in frontal patients is compromised if the lesion involves a specific part of the DLPFC, corresponding to A9. In this case, frontal patients do not take advantage of the categorical cue.
Materials and methods
Participant sample
Ten frontal lobe-damaged patients (FL) and 10 healthy control subjects (HC) were included in this study.
Subjects with frontal neoplastic lesions (low-grade gliomas) were recruited during a nine-month period from patients at the Neurosurgery Unit of the “Policlinico Maggiore” Hospital in Milan. Each patient was tested at least six months after surgery, concurrently with the control MRI scans.
The main inclusion criteria required a single primitive frontal lesion on the left prefrontal cortex. Patients had to be in treatment with anti-epileptic therapy and with no evidence of epileptic seizures in the past month. We considered the following exclusion criteria: brain lesions located in any site other than the left frontal lobe; neurodegenerative disease or any other neurological condition determining a history of cognitive decline; history of any other significant neurological or psychiatric disorder; non-neurological comorbidities that could potentially compromise the neuropsychological testing results. Patients had to obtain normal scores in MMSE [30], Token Test [31], Boston Naming Test [32]. Eleven FL were recruited. However, one female patient was excluded because of aphasia documented by significantly low scores in fluency testing and in the Token test. Therefore, the total number of FL patients eventually included in the study is ten, two males and eight females (average age = 48.7 ± 13.3 DS; average education 14.1 ± 3.8 DS).
Frontal patients were divided into two groups: 6 subjects with tumoral lesion sparing area 9 (Fn) and 4 subjects with lesion involving area 9 (F9).
For this purpose, three independent skilled operators were required to identify area 9 according to Petrides and Pandya criteria in the MRI sequences, evaluating whether it was spared by the lesion. The three operators’ judgements were unanimous, except for one FL patient whose circumstance was discussed until reaching an agreement. The neuropsychological testing was performed within 3 days before MRI.
Ten healthy control subjects (HC), matched to FL for age, gender, and years of education, were recruited for the experiment. Their medical history was negative for neuropsychiatric disorders and for the use of psychotropic drugs. Their performances in MMSE [30], Token Test [31] and Boston Naming Test [32] resulted within normal range.
MRI acquisition
MRI examination was performed by a 3 Tesla Scanner (Philips Achieva Eindhoven, The Netherlands), equipped with a 32-channel sensitivity encoding coil. DTI sequence was acquired as an axial, single-shot, echo planar imaging sequence with the following parameters: repetition time = 7723 ms, echo time = 70 ms, flip angle = 90°, voxel size = 2 × 2 × 2 mm3, multi-band factor = 2.64 diffusion directions using 1000 and 0 s/mm2 ad b-values. The DTI acquisition time was 5 min and 31 s/5′31″. The MR protocol also included volumetric turbo field-echo T1-weighted, and fluid-attenuated inversion recovery sequences, axial turbo spin-echo T2-weighted sequence, axial susceptibility-weighted sequence.
Neuropsychological assessment
All patients underwent a battery of neuropsychological tests [33,34,35,36,37,38,39,40,41], performed by a dedicated neuropsychologist (BZ), to investigate language, memory, executive functions, and attention. Each domain was explored through multiple tests (see Table 1), which proved to be sensitive in detecting cognitive impairment due to organic causes [42]. The raw scores were adjusted for age, education, and sex [43], with Italian normative data [33,34,35,36,37,38,39,40,41]. Mean and standard deviation of adjusted scores achieved by the patients on the neuropsychological battery tests are shown in Table 1.
The learning process was studied using two lists of words, designed by Faglioni and colleagues [44] specifically to evaluate category clustering ability (see Fig. 1).
Both lists consisted of nine disyllabic common nouns chosen from three semantic categories (animals, objects, and human body for List A; colors, materials, and clothing for list B); the nouns occur in a pseudo-random order such that no two words from the same category are next to each other. Words were balanced for frequency of use according to Bortolini and colleagues [45], across categories within each list. Imageability and familiarity ratings were equivalent in each list.
Subjects had to learn each list through fifteen study-recall trials, following Buschke and Fuld’s selective reminding technique [46]. The large number of trials enhances the likelihood of uncovering the categories within the list, a phenomenon anticipated to happen especially after several attempts both in the normal subject and, even more so, in the frontal patient. The constrained number of words in each list is meant to limit the duration of the test, as a higher cognitive demand (meaning a higher number of words) over a long period of time might overload sustained attention, thus interfering with the result.
The selective reminding technique, already used to investigate different clinical conditions using both categorically related and unrelated words, was preferred over the standard serial presentation of all the nine items before each recall trial because it allows to compensate for primacy and recency effects. Subjects were informed that the entire list would be presented on the first study trial, but only the words they had failed to recall would be reminded in each subsequent study trial; if they recalled the entire list, they would have to repeat it again on the next trial, and the examination would not stop until the 15th attempt. After that, the examiner read aloud the words at a rate of one word every two seconds, then he/she asked the subject to repeat all the words they could remember, regardless of the order of presentation. Unlimited time was allowed for recall so as not to penalize subjects with a long recall latency. The examiner recorded the order of the responses given at each recall trial.
All subjects were tested under two different verbal learning conditions (un-cued condition and cued condition) using two different lists.
In the un-cued condition, participants were not warned that a semantic structure was embedded in the list. In the cued condition, the examiner provided orally the names of the three categories by which subjects could group the nine items and then he/she wrote the category names on a piece of paper and gave it to the subjects, making sure they could see it for the whole duration of the test. Subjects were subsequently required to use categories during the recall. Lastly, the examiner began reading the items of the list.
The un-cued condition always preceded cued condition to avoid the effect of the cue on both tests. To avoid internal list effects, half of the subjects from each group studied List A in the un-cued condition, whereas the other half studied List B. In the following cued condition, each subject studied the remaining list.
Each learning test lasted no more than 30 min. All subjects performed other neuropsychological tests for 1 h between the two testing sessions.
Two main parameters were used to evaluate subjects’ performances in each list: Learning Score (LS) and Clustering Index (CI). LS constitutes the total number of recalled list words, whereas CI is calculated as the ratio between categorized words and total recalled words. A word is labeled as categorized if preceded or followed by other words pertaining to the same semantic category.
Mean and standard deviation of LS and CI for Fn, F9 and HC are shown in Table 2 and represented in Figs. 2 and 3. Average number of words recalled by frontal patients at each trial in the uncued and cued condition are shown in Figs. 4 and 5.
Learning performance of frontal patients with lesions sparing or involving area 9 and healthy control subjects. LS1 = Learning score in the un-cued condition, LS2 = Learning score in the cued condition, F9 = patients with lesions involving area 9, Fn = patients with lesions sparing area 9, HC = healthy control subjects
Semantic categorization ability of frontal patients with lesions sparing or involving area 9 and healthy control subjects. CI1 = Clustering Index in the un-cued condition, CI2 = Clustering Index in the cued condition, F9 = patients with lesions involving area 9, Fn = patients with lesions sparing area 9, HC = healthy control subjects
Statistical analyses
Mixed models were adopted to test the effect of Group, Condition and their interaction on the CI and LS, by addressing Subject as the cluster.
Both outcomes proved not to be Normally distributed, as indexed by significant Shapiro–Wilk’s statistics (ps < 0.001) and graphical inspections (i.e., histograms and quantile–quantile plots) on raw variables – the latter revealing heavily left-skewed and overdispersed distributions. Both the CI and the LS were thus reversed, by subtracting values from their theoretical maximum (i.e., 1 for the CI and 135 for the LS) – in order for them to be addressed as outcomes within generalized linear mixed models (GLMMs) assuming heavily right-skewed, overdispersed distributions [47]. More specifically, the reversed CI and LS – i.e., the Non-Categorization Index (NCI) and the number of unrecalled words (UWs) – were assumed to underlie a Gamma [48] and a Negative Binomial [49] distribution, respectively – as the former models continuous, and the latter frequency, data. Since the three groups were matched for age (F(2,17) = 0.01; p = 0.990), education (F(2,17) = 0.66; p = 0.529) and sex (χ2(2) = 1.67; p = 0.435), none of these variables was entered as a covariate into the abovementioned models. Results of the analysis were shown in Fig. 6.
The significance level was set at α = 0.05; within both the Gamma and the Negative Binomial GLMM, post-hoc comparisons were Bonferroni-corrected. Analyses were run via jamovi 2.3 (https://www.jamovi.org).
Results
The Gamma GLMM on the NCI revealed a significant main effect of Condition (χ2(1) = 40.62; p < 0.001), with the non-cued condition resulting in higher NCI values (M = 0.43; SE = 0.10) when compared to the cued one (M = 0.04; SE = 0.01), and Group (χ2(2) = 6.66; p = 0.036), whose decomposition revealed that F9 patients (M = 0.25; SE = 0.11) performed worse (p = 0.030) than HC (M = 0.07; SE = 0.02), with no other comparison yielding significance (ps ≥ 0.365). Moreover, the significant Group*Condition interaction (χ2(2) = 7.63; p = 0.022) (see Fig. 6) revealed that the cued condition selectively favored HC (cued: M = 0.01; SE = 0.01 vs. non-cued: M = 0.39; SE = 0.12; p < 0.001) and Fn patients (cued: M = 0.03; SE = 0.01 vs. non-cued: M = 0.41; SE = 0.16; p < 0.001), but not F9 patients (cued: M = 0.13; SE = 0.08 vs. non-cued: M = 0.48; SE = 0.23; p = 1).
As for the Negative Binomial GLMM on UWs, a main effect of Condition (χ2(2) = 19.28; p < 0.001) – with the non-cued condition overall resulting in a higher number of UWs (M = 17.3; SE = 6.07) when compared to the cued one (M = 6.91; SE = 1.39) – and Group (χ2(1) = 6.32; p = 0.042) emerged – with the only significant post-hoc comparison (p = 0.038) being that between F9 (M = 18.37; SE = 6.07) and HC (M = 6.91; SE = 1.5). At variance with the results on the NCI, the Group*Condition interaction on UWs was not significant (χ2(2) = 0.53; p = 0.767).
Performance on the neuropsychological battery tests did not differ between F9 and Fn, ruling out the influence of other cognitive disorders on the above comparisons. The ability of frontal patients to perform highly on the Auditory Verbal Learning Test indicates that their deficit is confined to the semantic organization of verbal material and does not involve other aspects of memory functioning.
Discussion
In the non-cued word list learning task, F9 recall fewer words and they are also less likely to group them into categories when compared to HC. However, Fn do not significantly differ from HC in terms of number of recalled words or propensity to spontaneously categorize items.
One may theorize that the identification of categories is quite demanding and could overload WM, slowing down HC’ efficiency in the subsequent trials. Instead, frontal patients’ WM – even if generally impaired because of the lesion – is not overloaded by the identification of categories since patients tend to neglect them. This could explain why the differences in performance between patients and controls are reduced, remaining evident only for F9. However, in line with Alexander and colleagues’ [5] observation, A9 would play a critical role in learning process.
Even when given the strategic cue, F9 patients exhibit a decrease in word acquisition and categorization, while Fn patients show no difference from HC. Previous studies showed that strategic cues improve learning in frontal patients, who may reach normal scores [6, 8, 10]. However, these studies did not design subgroups according to the frontal lesion site, which is a relevant factor for subjects’ performance. In our study, this subdivision showed that, in the case of A9 involvement, the strategic cue does not improve learning.
It is remarkable that, compared to HC, F9 do not benefit from the semantic cue in organizing recalled words, unlike Fn who, similarly to HC, significantly improve when transitioning from the no-cued to the cued condition. This confirms the crucial role played by area 9 in the implementation of learning strategies, as suggested by TMS evidence [27].
The small sample size and the lack of specific neuroimaging data impose some caution in the interpretation of the results; however, the significance of the statistical comparisons in line with the experimental hypothesis encourages further studies on this topic.
In summary, our results show that frontal patients’ verbal learning defect is due to impaired categorical strategy implementation and that this ability can be specifically attributed to the left area 9.
Data availability
Datasets cannot be made publicly available on both ethical and legal grounds but may be made available upon reasonable request of interested researchers to the Corresponding Author, who will in turn forward a request for a data transfer agreement to the relevant Ethical Committee.
Change history
20 May 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10072-024-07596-4
Abbreviations
- FL:
-
Frontal lobe-damaged patients
- Fn:
-
Patients with lesion sparing area 9
- F9:
-
Patients with lesion involving area 9
- HC:
-
Healthy control
- PFC:
-
Prefrontal cortex
- VLPFC:
-
Ventrolateral prefrontal cortex
- DLPFC:
-
Dorsolateral prefrontal cortex
- STM:
-
Short-term memory
- WM:
-
Working memory
- BA9:
-
Area 9 according to Brodmann’s classification
- A9:
-
Area 9 according to Petrides and Pandya’s classification
- LS:
-
Learning Score
- CI:
-
Clustering Index
- NCI:
-
Non-Categorization Index
- UWs:
-
Unrecalled words
References
Bousfield WA (1953) The occurrence of clustering in the recall of randomly arranged associates. J Gen Psychol 49(2):229–240. https://doi.org/10.1080/00221309.1953.9710088
Jenkins J, Russell W (1952) Associative clustering during recall. Psychol Sci Public Interest 47(4):818–821. https://doi.org/10.1037/h0063149
Tulving E, Osler S (1967) Transfer effects in whole/part free-recall learning. Can J Psychol 21(3):253–262. https://doi.org/10.1037/h0082979
Mandler G, Pearlstone Z (1966) Free and constrained concept learning and subsequent recall. J Verbal Learning Verbal Behav 5(2):126–131. https://doi.org/10.1016/S0022-5371(66)80005-1
Alexander MP, Stuss D, Gillingham S (2009) Impaired list learning is not a general property of frontal lesions. J Cogn Neurosci 21(7):1422–1434. https://doi.org/10.1162/jocn.2009.21094
Gershberg FB, Shimamura AP (1995) Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia 33(10):1305–1333. https://doi.org/10.1016/0028-3932(95)00103-A
Stuss D, Alexander M, Palumbo C, Buckle L, Sayer L, Pogue J (1994) Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology 8(3):355–373. https://doi.org/10.1037/0894-4105.8.3.355
Hirst W, Volpe BT (1988) Memory strategies with brain damage. Brain Cogn 8(3):379–408. https://doi.org/10.1016/0278-2626(88)90060-7
Kopelman MD, Stanhope N (1998) Recall and recognition memory in patients with focal frontal, temporal lobe and diencephalic lesions. Neuropsychologia 36(8):785–796. https://doi.org/10.1016/S0028-3932(97)00167-X
Daum I, Schugens MM, Spieker S, Poser U, Schönle PW, Birbaumer N (1995) Memory and skill acquisition in Parkinson’s disease and frontal lobe dysfunction. Cortex 31(3):413–432. https://doi.org/10.1016/S0010-9452(13)80057-3
Eslinger PJ, Grattan LM (1994) Altered serial position learning after frontal lobe lesion. Neuropsychologia 32(6):729–739. https://doi.org/10.1016/0028-3932(94)90032-9
Janowsky JS, Shimamura AP, Squire LR (1989) Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 27(8):1043–1056. https://doi.org/10.1016/0028-3932(89)90184-X
Jetter W, Poser U, Freeman RB, Markowitsch HJ (1986) A verbal long term memory deficit in frontal lobe damaged patients. Cortex 22(2):229–242. https://doi.org/10.1016/S0010-9452(86)80047-8
Della Rocchetta AI (1986) Classification and recall of pictures after unilateral frontal or temporal lobectomy. Cortex 22(2):189–211. https://doi.org/10.1016/S0010-9452(86)80045-4
Della Rocchetta AI, Milner B (1993) Strategic search and retrieval inhibition: The role of the frontal lobes. Neuropsychologia 31(6):503–524. https://doi.org/10.1016/0028-3932(93)90049-6
Vilkki J, Servo A, Surma-aho O (1998) Word list learning and prediction of recall after frontal lobe lesions. Neuropsychology 12(2):268–277. https://doi.org/10.1037/0894-4105.12.2.268
Alexander MP, Stuss DT, Fansabedian N (2003) California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain 126(Pt 6):1493–1503. https://doi.org/10.1093/brain/awg128
Milner B (1972) Disorders of learning and memory after temporal lobe lesions in man. Neurosurgery 19(suppl 1):421–446. https://doi.org/10.1093/neurosurgery/19.CN_suppl_1.421
Guidali G, Pisoni A, Bolognini N, Papagno C (2019) Keeping order in the brain: The supramarginal gyrus and serial order in short-term memory. Cortex 119:89–99. https://doi.org/10.1016/j.cortex.2019.04.009
Petrides M (1996) Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B, Biol Sci 351(1346):1455–1462. https://doi.org/10.1098/rstb.1996.0130
Petrides M (2005) Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond, B, Biol Sci 360(1456):781–795. https://doi.org/10.1098/rstb.2005.1631
Petrides M (1995) Functional organization of the human frontal cortex for mnemonic processing. Ann N Y Acad Sci 769:85–96. https://doi.org/10.1111/j.1749-6632.1995.tb38133.x
Petrides M (1994) Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J (eds) Handbook of neuropsychology. Elsevier, pp 59–82
Owen AM (2000) The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp Brain Res 133(1):33–43. https://doi.org/10.1007/s002210000398
Blumenfeld RS, Ranganath C (2006) Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci 26(3):916–925. https://doi.org/10.1523/JNEUROSCI.2353-05.2006
Balconi M (2013) Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull 29(3):381–389. https://doi.org/10.1007/s12264-013-1309-z
Sandrini M, Cappa SF, Rossi S, Rossini PM, Miniussi C (2003) The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J Cogn Neurosci 15(6):855–861. https://doi.org/10.1162/089892903322370771
Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73(1):59–86. https://doi.org/10.1002/cne.900730106
Petrides M, Pandya DN (1999) Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns: Dorsolateral prefrontal cortex in human and monkey. Eur J Neurosci 11(3):1011–1036. https://doi.org/10.1046/j.1460-9568.1999.00518.x
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. https://doi.org/10.1016/0022-3956(75)90026-6
De Renzi A, Vignolo LA (1962) Token test: A sensitive test to detect receptive disturbances in aphasics. Brain 85:665–678. https://doi.org/10.1093/brain/85.4.665
Kaplan E, Goodglass H, Weintraub S (1983) The Boston naming test. Lea & Febiger, Philadelphia
De Renzi E, Faglioni P (1978) Normative data and screening power of a shortened version of the token test. Cortex 14(1):41–49. https://doi.org/10.1016/s0010-9452(78)80006-9
Catricalà E, Della Rosa PA, Ginex V, Mussetti Z, Plebani V, Cappa SF (2013) An Italian battery for the assessment of semantic memory disorders. Neurol Sci 34(6):985–993. https://doi.org/10.1007/s10072-012-1181-z
Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987) Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8(6):539–548. https://doi.org/10.1007/BF02333660
Carlesimo GA, De Risi M, Monaco M et al (2014) Normative data for measuring performance change on parallel forms of a 15-word list recall test. Neurol Sci 35:663–668. https://doi.org/10.1007/s10072-013-1573-8
Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) A short version of the Stroop test: normative data in an Italian population sample. Nuova Riv Neurol 12:111–115
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail Making Test: Normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309. https://doi.org/10.1007/BF01997792
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci 34:749–754. https://doi.org/10.1007/s10072-012-1130-x
Novelli G, Papagno C, Capitani E, Laiacona M, Vallar G, Cappa SF (1986) Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr. 47:477–506
Spinnler H (1987) Italian standardization and classification of Neuropsychological tests. The Italian Group on the Neuropsychological Study of Aging. Ital J Neurol Sci. Suppl 8:1–120
Lezak MD (1994) Domains of behavior from a neuropsychological perspective: the whole story. Neb Symp Motiv 41:23–55
Capitani E (1997) Normative data and neuropsychological assessment. Common problems in clinical practice research. Neuropsychol Rehabil. 7:295–310. https://doi.org/10.1080/713755543
Faglioni P, Saetti MC, Botti C (2000) Verbal learning strategies in Parkinson’s disease. Neuropsychology 14(3):456–470. https://doi.org/10.1037//0894-4105.14.3.456
Bortolini U, Tagliavini U, Zampolli A (1972) Lessico di frequenza della lingua italiana contemporanea. Garzanti, Milano
Buschke H, Fuld PA (1974) Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24(11):1019–1025. https://doi.org/10.1212/WNL.24.11.1019
Aiello EN, Depaoli EG, Gallucci M (2020) Usability of the negative binomial model for analyzing ceiling and highly-inter-individually-variable cognitive data. Neurol Sci 41:S273–S274. https://doi.org/10.1007/s10072-020-04753-3
Husak GJ, Michaelsen J, Funk C (2007) Use of the gamma distribution to represent monthly rainfall in Africa for drought monitoring applications. Int J Climatol 27(7):935–944. https://doi.org/10.1002/joc.1441
VerHoef JM, Boveng PL (2007) Quasi-Poisson vs. negative binomial regression: how should we model overdispersed count data? Ecology 88(11):2766–2772. https://doi.org/10.1890/07-0043.1
Acknowledgements
The authors wish to thank all study participants.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. No funding was received towards this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests.
Ethical approval and Informed consent
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All participants have given their written informed consent, and the study protocol was reviewed and approved by the Territorial Ethics Committee (CET) Lombardia 3, meeting of 08.11.2023, consensus 4147_S_P, ID 4147.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The abstract text has been revised.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cocuzza, A., Bertani, G., Conte, G. et al. Verbal learning in frontal patients: area 9 is critical for employing semantic strategies. Neurol Sci (2024). https://doi.org/10.1007/s10072-024-07569-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10072-024-07569-7