Abstract
Individuals suffering from long-COVID can present with “brain fog”, which is characterized by a range of cognitive impairments, such as confusion, short-term memory loss, and difficulty concentrating. To date, several potential interventions for brain fog have been considered. Notably, no systematic review has comprehensively discussed the impact of each intervention type on brain fog symptoms. We included studies on adult (aged > 18 years) individuals with proven long- COVID brain-fog symptoms from PubMed, MEDLINE, Central, Scopus, and Embase. A search limit was set for articles published between 01/2020 and 31/12/2023. We excluded studies lacking an objective assessment of brain fog symptoms and patients with preexisting neurological diseases that affected cognition before COVID-19 infection. This review provided relevant information from 17 studies. The rehabilitation studies utilized diverse approaches, leading to a range of outcomes in terms of the effectiveness of the interventions. Six studies described noninvasive brain stimulation, and all showed improvement in cognitive ability. Three studies described hyperbaric oxygen therapy, all of which showed improvements in cognitive assessment tests and brain perfusion. Two studies showed that the use of Palmitoylethanolamide and Luteolin (PEA-LUT) improved cognitive impairment. Noninvasive brain stimulation and hyperbaric oxygen therapy showed promising results in the treatment of brain fog symptoms caused by long-COVID, with improved perfusion and cortical excitability. Furthermore, both rehabilitation strategies and PEA-LUT administration have been associated with improvements in symptoms of brain fog. Future studies should explore combinations of interventions and include longer follow-up periods to assess the long-term effects of these treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction & background
More than 774,075,242 coronavirus disease 2019 (COVID-19) cases have been reported by the World Health Organization (WHO). As the world transitions into a postpandemic era, COVID-19 continues to persist in the form of various variants and subvariants [1]. Approximately 10–35% of COVID-19 survivors experience persistent symptoms such as fatigue, dyspnea, chest pain, cough, depression, anxiety, posttraumatic stress disorder, memory loss, and difficulty concentrating [2]. The National Institute for Health and Care Excellence (NICE) guidelines characterize the persistence of symptoms as long-COVID. According to these guidelines, long-COVID involves symptoms that persist for > 12 weeks (3 months) and cannot be attributed to an alternative diagnosis [3]. Moreover, one of the three patients with COVID-19 will be diagnosed with neurological symptoms within 6 months of infection [4]. Neurological symptoms characterized by impaired intellectual functions in individuals with long-COVID are collectively referred to as "brain fog," which encompasses a range of cognitive impairments, such as confusion, short-term memory loss, and difficulty concentrating [5, 6]. The mechanism underlying how long-COVID causes brain fog symptoms is not entirely understood. However, evidence indicates that COVID-19 may invade the brain through various possible routes. This invasion triggers neuroinflammatory processes that can activate cells such as astrocytes and microglia. These processes may contribute to the neurological symptoms observed in long-COVID patients [6]. Regarding the factors influencing brain fog caused by long-COVID, studies have shown that female patients and those who experienced a milder course of acute COVID tend to be more susceptible to developing brain fog [7].
The significance of cognitive function cannot be overstated because it plays a crucial role in our daily lives. Any impairment in cognitive function can have a severe impact on quality of life. Studies indicate that individuals experiencing brain fog often suffer from decreased occupational function, making it challenging for them to resume their normal occupations [8,9,10]. Furthermore, brain fog has been linked to depression and poor sleep quality [11, 12]. Given the adverse impact on quality of life and the widespread occurrence of brain fog, exploring interventions to improve quality of life and providing treatment are important areas of research.
To date, several potential interventions for brain fog have been considered, including noninvasive brain stimulation, hyperbaric oxygen therapy, and traditional and nontraditional rehabilitation approaches. Notably, no systematic review has comprehensively discussed the impact of each intervention type on brain fog symptoms. Therefore, the goal of this systematic review was to explore the effects of different intervention types on brain fog symptoms in those suffering from long-Covid.
Methods
This study used the following methodological framework in conjunction with the extended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for systemic reviews [13]. The study protocol was preregistered on the International Prospective Register Reviews (PROSPERO; CRD42024502977). The primary aim of this study was to describe interventions for brain fog caused by long-COVID. Due to the innovative nature of this review, the included studies applied diverse methodologies for documenting improvements and diagnosing brain fog symptoms, employing various tests to detect mild cognitive impairments. Additionally, considering the range of papers providing quantitative outcomes, conducting a meta-analysis might not be feasible. Therefore, narrative synthesis was the most appropriate for the different types of studies we found.
Search strategy and selection criteria
This systematic review will encompass studies focusing on individuals who have exhibited confirmed brain fog symptoms attributed to long-COVID. from PubMed, Central, Scopus and Web of Science to establish an extensive pool of helpful information regarding brain fog symptoms. We intentionally set a search limit for the period from January 1, 2020, to December 31, 2023. This time frame was chosen because COVID-19 cases began to emerge in December 2019, marking the onset of the pandemic. The following search terms were used: “Post-Acute COVID-19 Syndrome” OR "COVID-19″ AND "Mental Fatigue,” OR "Cognitive Dysfunction,” OR "therapy,” OR "Cognitive Training,” OR "Hyperbaric Oxygenation,” OR "Transcranial Magnetic Stimulation”.
Inclusion and exclusion criteria
We included studies with adult populations (≥ 18 years) exhibiting brain fog symptoms at least four weeks post-COVID-19 infection. Studies were required to provide specific descriptions of brain fog symptoms rather than relying on broad terminology such as 'cognitive impairment'. Furthermore, additional studies are needed to detail interventions aimed at addressing brain fog symptoms. We excluded studies lacking objective assessments of brain fog, those with unclear diagnoses, and those involving patients with preexisting neurological conditions affecting cognition. Additionally, we excluded non-English studies, systematic reviews, and meta-analyses.
Data extraction and quality assessment
Screening and data extraction were performed by four independent reviewers (A.G., T.L., S.S., and L.L.). Any disagreements were discussed, and a consensus was reached by four reviewers. For studies that reported on the control and patient groups, only patient data were extracted and used, as per the decision to combine both clinical trials and observational studies. The following data were retrieved for each article: first author's name, location, publication time, study type, number of long COVID-19 patients, outcome measure, intervention modality, duration of treatment, number of sessions, adverse effects, primary study findings, and secondary outcomes. Four authors (A.G., T.L., S.S., and L.L.) independently extracted information from the full texts of the 16 selected studies. Inconsistencies between the reviewers were resolved through consultation with a senior reviewer (Y.S.).
For quality assessment, two authors (A.G. and T.L.) independently assessed (1) the criteria for the diagnosis of COVID-19, (2) the duration of the intervention methods used for assessing brain-fog symptoms caused by long-COVID, and (3) the scoring system used to assess brain-fog symptoms.
Risk of bias assessment
Risk of bias assessment for cohort studies was performed using the Newcastle–Ottawa Scale (NOS) (Table 1), and for randomized clinical trials (RCTs), risk of bias was assessed using the revised Cochrane risk-of-bias (ROB2) tool for RCTs (Fig. 1). In evaluating the included case reports, case series, and pilot studies, a comprehensive approach was used to assess the quality and reliability of the evidence presented. Each case report was scrutinized for clarity of reporting, objectivity of information, adherence to diagnostic criteria, appropriateness of treatment interventions, and transparency in outcome measures. Special attention was given to alternative explanations for the observed clinical findings, ethical considerations such as patient consent and confidentiality, and disclosures of conflicts of interest. Furthermore, the generalizability of the reported cases to broader clinical practice was considered, along with the educational value they provided. For pilot studies, particular emphasis was placed on methodological rigor, including clarity of research objectives, appropriateness of study design, transparency in data collection and analysis, and consideration of potential biases. Overall, this comprehensive approach enabled a thorough assessment of the strengths and limitations of the individual case reports, case series, and pilot studies included in the review, contributing to a nuanced understanding of the evidence base for the evaluated interventions and clinical phenomena.
Results
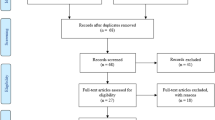
In total, 5770 articles were reviewed after the removal of duplicates, 2613 articles were screened for titles and abstracts, and out of them, 287 articles met the criteria. These studies provided information on possible interventions to treat neurocognitive deficits or long-COVID. Subsequently, the full texts of these articles were evaluated following the inclusion and exclusion criteria that were issued above; after quality assessment, 17 studies were included in this review [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. Figure 2 shows the flow diagram detailing the review process and study selection based on the PRISMA flow chart.
Characteristics of the results
Table 2 presents the characteristics and findings of the included studies. The sample sizes ranged from one to 208. The earliest publication date was 01/11/2021, while the latest was 20/03/2024. Thirteen countries were included in the review: the USA, Norway, the United Kingdom, Spain, Italy, Germany, Taiwan, Israel, Poland, the United Arab Emirates, Brazil, France, and Japan. The following study types were included: cohort, observational, exploratory, pilot study, clinical trial, case report, and case series. A total of 806 patients were diagnosed with COVID-19. A variety of assessment tools were employed to evaluate brain fog, including the Wechsler Memory Scale (WMS), Third Edition, Montreal Cognitive Assessment (MoCA), Test Battery for Attention (TAP), Auditory Verbal Learning Test (AVLT), Wechsler Adult Intelligence Scale (WAIS4)-Fourth Edition, Mini-Mental State Examination (MMSE), NeuroTrax computerized testing battery, CANTAB cognitive research software, Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS), Cognitive Assessment Battery (CAB), Psychology Experiment Building Language (PEBL), Prospective–Retrospective Memory Questionnaire (PRMQ), Fatigue Severity Scale (FSS), and Perceived Deficits Questionnaire-Depression 5-item (PDQ-D-5).
Several intervention modalities, such as rehabilitation (individualized psychological intervention of cognitive and cognitive behavioral therapy, personalized computerized cognitive training, aerobic exercise training, body awareness training, breathing therapy, mobile application, and mindfulness-based interventions), noninvasive brain stimulation (transcranial magnetic stimulation, theta burst stimulation, transcranial direct current stimulation, transcranial alternating current stimulation, and photobiomodulation), hyperbaric oxygen therapy and pharmacological therapy (palmitoylethanolamide and luteolin (PEA-LUT)), have been studied (Table 2).
Duration of treatment
In this review, we examined the median time for treatment and the number of sessions required for each type of intervention. Our analysis revealed notable variations in both aspects across interventions. In terms of the median time for treatment, noninvasive brain stimulation emerged as the most expedient, demonstrating a median treatment duration of 13.5 days. This was followed by rehabilitation, which exhibited a median treatment duration of 32.5 days. Moreover, the median duration of hyperbaric oxygen therapy treatment was 56 days, while pharmacological intervention necessitated the longest median duration of treatment, with a duration of 75 days. Moreover, when examining the number of sessions needed, we noticed different patterns across interventions. Noninvasive brain stimulation required the fewest sessions, with a median of 12 sessions, while hyperbaric oxygen therapy required a median of 40 sessions. Table 3 summarizes the median and mean values for the number of sessions and treatment duration associated with each intervention for brain fog induced by long-COVID.
Adverse effects
Regarding adverse effects, noninvasive brain stimulation and hyperbaric oxygen therapy revealed mild adverse effects. The case report by Chang et al. presented a 30-year-old female with persistent anxiety, depression, insomnia, and brain fog symptoms for eight weeks after COVID-19 infection. Chang et al. used accelerated theta burst stimulation of the bilateral dorsolateral prefrontal cortex using an Apollo transcranial magnetic stimulation therapy system as an intervention. The adverse effects of the intervention were dizziness and headache; however, they were transient and resolved after treatment [14]. Noda et al. conducted a case series of 23 patients with long-term COVID-19. The intervention protocol consisted of one session of intermittent theta burst stimulation for the dorsolateral prefrontal cortex and one session of low-frequency repetitive transcranial magnetic stimulation for the right lateral orbitofrontal cortex with one transcranial magnetic stimulation treatment per day [26]. The adverse effect of the intervention was scalp pain at the stimulation site, which was reported by 4 out of 23 patients. In a clinical trial conducted by Zilberman-Itskovich et al., 73 patients with long COVID-19 were identified, with 37 and 36 patients in the intervention and control groups, respectively. The intervention group received 40 daily sessions of hyperbaric oxygen therapy. The following adverse effects were reported: barotrauma (n = 4), ear pain without barotrauma (n = 1), palpitations (n = 3), headache (n = 1) and fever (n = 1) [27]. These effects were generally manageable and did not impede the overall efficacy of the interventions. Conversely, rehabilitation and pharmacological treatment had no adverse effects.
Brain-fog outcomes
Rehabilitation
figSix studies described the use of rehabilitation as a treatment modality [15, 18, 19, 22, 24, 25]. Each study employed a slightly different rehabilitation approach, resulting in varied outcomes regarding the success of the interventions. Table 4 illustrates the specific rehabilitation strategies implemented in each study. Four studies revealed enhancements in brain fog symptoms [19, 22, 24, 25], whereas two studies reported no significant improvement in brain fog symptoms [15, 18]. Kupferschmitt et al. performed a prospective cohort study of 80 post-COVID-19 patients who underwent multimodal rehabilitation. The multimodal rehabilitation concept included cognitive behavioral therapy and TAP, followed by cognitive training in a group setting (2 × 50 min/week) and in an individual setting (as needed), individualized aerobic exercise training, body awareness training, breathing therapy, relaxation techniques, and social counseling. The duration of rehabilitation was five weeks [15]. Kupferschmitt et al. indicated that depressive symptoms decreased to subclinical levels, as assessed by the Patient Health Questionnaire-9, both before admission and after discharge. However, cognitive deficits persist throughout the rehabilitation process, as measured by TAP scores [15]. Samper-Pardo et al. developed a mobile application named the ReCOVery app for their clinical trial involving 100 patients with long COVID-19. The intervention group utilized the mobile application in conjunction with the treatment-as-usual methods recommended by their general practitioners for three months [18]. The study's findings suggested that the use of the ReCOVery app for three months did not significantly enhance quality of life in patients with long-COVID [18]. In contrast to conventional rehabilitation methods, unconventional rehabilitation strategies have been explored in certain studies [18, 24, 25]. One such investigation by Hausswirth et al. involved a parallel randomized controlled trial in which 34 long-term COVID-19 patients were divided randomly into an intervention group (n = 17) and a control group (n = 17), with an additional 15 healthy individuals serving as a standard comparison group. The intervention cohort engaged in the Rebalance® Program, which emphasizes mindfulness-based interventions, encompassing ten sessions of 30 min each over four weeks [25]. Cognitive enhancements were gauged using the PEBL platform, revealing noticeable improvements in the intervention group, whereas the control group showed no significant cognitive enhancements. Moreover, the benefits of the mindfulness-based intervention appeared to persist, with cognitive improvements becoming more pronounced a week following the neuro-meditation intervention [25]. Additionally, Duñabeitia et al. studied 73 post-COVID-19 patients suffering from brain fog. Their objective was to mitigate these symptoms via personalized computerized cognitive training. Participants were assessed initially and after completing at least 10 training sessions across 8 weeks. The training regimen was customized to the individual cognitive profiles of the patients, as determined by the CAB, utilizing proprietary Individualized Training System software [24]. Posttraining, Duñabeitia et al. reported uniform improvements across various cognitive areas in posttest evaluations compared to the initial assessments, signifying enhancements following personalized computerized cognitive training [24].
Noninvasive brain stimulation
Studies described the use of noninvasive brain stimulation as a treatment modality [14, 16, 21, 23, 26, 28]. Noninvasive brain stimulation involves a variety of techniques. In our review, we describe theta burst stimulation [14, 26], transcranial magnetic stimulation [16, 26], transcranial direct current stimulation [21], transcranial alternating current stimulation [23], and photobiomodulation [28]. Despite the use of various types of noninvasive brain stimulation, all studies have demonstrated improvements in brain fog symptoms [14].
Hyperbaric oxygen therapy
Three studies described the use of hyperbaric oxygen therapy as the treatment modality [20, 27, 29]. In all three studies, there was an improvement in perfusion (assessed by perfusion magnetic resonance imaging) and a reduction in brain fog symptoms.
Pharmacological
Two pharmacological studies met the inclusion criteria [17, 30]. De Luca et al. performed a clinical trial in which 69 long-term COVID-19 patients were divided into three groups: group 1, recurrent PEA-LUT plus olfactory training group (n = 43); group 2, recurrent pea-lut alone group (n = 16); and group 3, individuals who were exposed to olfactory training; these patients continued olfactory treatment while receiving PEA-LUT (n = 10). Cognitive impairment was assessed using the MMSE. Mental clouding showed a statistically significant reduction in severity between baseline and three months after treatment in groups 1 and 3. Group 2 showed no significant reduction in severity between baseline and three months after treatment [17]. Cenacchi et al. conducted an exploratory study comparing 26 patients treated with PEA-LUT to 15 who did not. They reported significant enhancements in the PRMQ and MoCA scores among those receiving PEA-LUT. Additionally, a secondary analysis of a subset of patients who received both PEA-LUT and corticosteroids (n = 7) versus those who received PEA-LUT alone (n = 19) revealed no significant difference in outcomes between the two groups [30].
Secondary outcome
In addition to treating brain fog symptoms, each intervention managed to treat other comorbidities of the patients, more specifically long-COVID symptoms that are not brain fog.
Rehabilitation
One study showed that throughout rehabilitation depressive symptoms decreased to a subclinical level [15] through the assessment of the PHQ-9.
Noninvasive brain stimulation
The following improvements in symptoms were observed across various studies: neuropsychiatric manifestations (anxiety, depression) [14], chronic fatigue [16], insomnia [14], and visual field recovery [23]. Theta burst stimulation [14], low-frequency repetitive transcranial magnetic stimulation [16] and transcranial alternating current stimulation [23] were used.
Hyperbaric oxygen therapy
The following outcomes were observed across various studies: improvement in physical capacity (VO2 increased) and lung function (FVC, FEV, PEF) [20]; depression, anxiety, sleep, and pain interference symptoms [27]; and fatigue [20, 27, 29].
Pharmacological
Improvement in parosmia was observed alongside improvement in brain fog symptoms [17]. Table 5 summarizes the secondary outcomes and adverse effects for each intervention.
Discussion
This systematic review aimed to explore possible interventions for brain fog symptoms in long-COVID patients. Our review identified four main approaches: rehabilitation, noninvasive brain stimulation, hyperbaric oxygen therapy and PEA-LUT. While we did encounter several pharmacological studies during our search, they were not included in our analysis because they did not utilize formal cognitive assessment tools.
The rehabilitation approach exhibited the greatest variability among the three interventions. This diversity in rehabilitation methods has resulted in mixed outcomes across studies assessing this intervention approach. Two of the six studies [15, 18] demonstrated that rehabilitation did not yield improvements or that the cognitive assessment scores did not surpass those of the control group. There are several potential reasons why certain studies have failed to produce significant results. Both studies lacked personalized treatment; they implemented a multimodal approach in which all patients underwent the same interventions without considering individual symptoms or preferences. In contrast, studies focusing on personalized treatment have demonstrated improvements in cognitive assessment scores [19, 24]. Studies have shown that individualized rehabilitation approaches can lead to improvements in patients with cognitive impairment [31,32,33]. The study by Samper-Pardo et al. that used telerehabilitation proposed that the lack of results could be due to the participants not significantly using the mobile application or allowing it to be an effective tool. Pardo et al. reported that only 25% of the participants made significant use of the mobile application, indicating low adherence toward the mobile application. The mean age of the participants in the study by Samper-Pardo et al. was 48.28 years. Mizrachi et al. indicated that individuals above the age of 50 may encounter difficulties with the use of technology in the healthcare field [34]. Furthermore, in the study by Braga et al., out of the 208 patients enrolled, only 133 (63.9%) actively participated in the rehabilitation program by attending at least two out of the four scheduled meetings. A significant portion, 47 (22.6%), did not attend any meetings, while 28 (13.5%) attended only one. This pattern of low adherence to the rehabilitation program is consistent with findings from the study by Samper-Pardo et al. Braga et al. also noted that patients who did not engage in psychoeducational groups and reported no use of compensatory strategies had worse average total BNIS scores. These results underscore the importance of high adherence to the rehabilitation approach and offer another explanation for the mixed results. Regarding the improvement of brain fog symptoms in other rehabilitation programs, several studies support these findings. In a randomized controlled trial conducted by Nauta et al., individuals with multiple sclerosis and cognitive impairment underwent cognitive rehabilitation and mindfulness treatment. This study revealed improvements in mindfulness and cognitive rehabilitation. However, after six months of treatment, cognitive rehabilitation showed benefits only for personalized cognitive goals, while mindfulness demonstrated benefits only for processing speed [35]. A meta-analysis by Hill et al. regarding computerized cognitive training in older adults with mild cognitive impairment or dementia showed that computerized cognitive training was an effective treatment option for mild cognitive impairment [36].
Although noninvasive brain stimulation has been used in a variety of approaches, successful treatment approaches for brain fog symptoms have been identified. Currently, noninvasive brain stimulation treatment is primarily used in rehabilitation for conditions such as stroke, spinal cord injury, traumatic brain injury, and multiple sclerosis [37]. It has also shown efficacy in treating neuropsychiatric manifestations, particularly refractory depression [38]. The findings of improvement in brain-fog symptoms were supported by a meta-analysis conducted by Wang et al. This meta-analysis focused on noninvasive brain stimulation as an intervention for mild cognitive impairment and Alzheimer's disease. They found that noninvasive brain stimulation had a significant effect on global cognition, and the use of low-frequency repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex improved memory function [39]. Noninvasive brain stimulation treatment primarily involves targeted neurostimulation of the dorsolateral prefrontal cortex and left orbitofrontal cortex. This approach was chosen because these regions are not only responsible for cognitive function but also because they can be directly and indirectly damaged by COVID-19 infection [40]. Noninvasive brain stimulation can facilitate stimulation of the dorsolateral prefrontal cortex, leading to improvements in neural rhythms, including theta and gamma amplitude coupling, which is also related to cognitive function and may even lead to enhanced neuroplasticity [41, 42]. The theory behind the success of these treatments is complex and not completely understood; however, one such example is cortical excitability and neuroplasticity. Cortical excitability refers to the intrinsic responsiveness of neurons in the cerebral cortex to excitatory and inhibitory inputs, which can be modulated by noninvasive brain stimulation [43]. These techniques induce changes in neuronal membrane potentials, synaptic efficacy, and neurotransmitter release, consequently altering the excitability of targeted brain regions. Concurrently, noninvasive brain stimulation treatments also facilitate neuroplasticity, the brain's ability to reorganize its structure and function in response to external stimuli or experiences. Neuroplastic changes induced by noninvasive brain stimulation involve synaptic remodeling, dendritic growth, and alterations in neural network connectivity, contributing to the adaptive responses observed following stimulation. The interplay between cortical excitability and neuroplasticity underlies the efficacy of noninvasive brain stimulation treatments across various neurological and neuropsychiatric conditions [44]. By enhancing cortical excitability and promoting neuroplasticity, noninvasive brain stimulation interventions harness the inherent plasticity mechanisms of the brain to promote recovery, alleviate symptoms, and improve cognitive function in affected individuals.
Hyperbaric oxygen therapy involves the delivery of 100% oxygen at environmental pressures exceeding one atmosphere. This process significantly increases the partial pressure of oxygen in the blood and tissues beyond what is achievable with standard oxygen supplementation [45]. Currently, it is acknowledged as an effective treatment method for a range of brain injuries [46, 47]. A study conducted by Chen et al. recruited patients with Alzheimer's disease and amnestic mild cognitive impairment for hyperbaric oxygen therapy, consisting of 40 min of treatment daily for 20 days. The results of the study showed that hyperbaric oxygen therapy significantly improved cognitive function, as assessed by the MMSE and MoCA [48]. This finding is consistent with the results of the studies included in our review. Hyperbaric oxygen therapy is an effective treatment for brain fog symptoms for several reasons. The common reason is the improvement in tissue oxygenation; moreover, improvement in tissue oxygenation was suggested to be a supportive therapy for COVID-19 [49]. Studies have indicated that hyperbaric oxygen therapy not only increases tissue oxygenation but also affects oxygen and pressure-sensitive genes, thereby promoting regenerative processes, such as stem cell proliferation and neurogenesis [50, 51]. This suggests that hyperbaric oxygen therapy may induce neuroplasticity and subsequently improve cognitive function [52,53,54].
Both studies investigating PEA-LUT reported positive outcomes for the use of this drug. The primary rationale for the effectiveness of PEA-LUT is attributed to its anti-inflammatory and neuroprotective effects, positioning it as an antagonist of neuroinflammation [55]. PEA, an innate component of the N-acylethanolamine family found in numerous tissues, including the brain, is synthesized in response to stress to restore tissue equilibrium. The success of PEA in ameliorating cognitive deficits may be due to its ability to inhibit the nuclear factor-κB (NF-κB) pathway by activating PPAR-α receptors [56], thereby dampening brain inflammation. This action is vital for addressing cognitive dysfunctions such as memory issues and brain fog. PEA’s neuroprotection potentially shields neurons against inflammatory harm, which is pertinent for long-COVID neurological symptoms [57]. Furthermore, PEA augments anandamide function—a neurotransmitter that modulates pain, appetite, and memory—by interacting with the cannabinoid-like receptors GPR55 and GPR119 [58], possibly restoring compromised neurotransmission in long-COVID cognitive disorders. By reducing oxidative stress and modifying inflammatory pathways, PEA may contribute to the alleviation of cognitive symptoms such as brain fog, underscoring its therapeutic promise for symptom management [59].
The mechanism behind brain fog symptoms post-COVID-19 is not completely understood. There are a variety of hypotheses. One prevailing hypothesis suggests that COVID-19 infects cells in the central nervous system through ACE2 receptors, particularly astrocytes, which are abundant in the central nervous system. When infected, astrocytes may modify their metabolic pathways, potentially causing harm to neighboring neurons, as astrocytes provide support to neurons. This disruption may explain the symptoms observed in individuals experiencing brain fog [60]. Another theory suggests that microglia are activated, possibly triggered by an entry point from the hypothalamus. The activation of these microglia can lead to the release of proinflammatory molecules [61]. Additionally, COVID-19 may worsen oxidative stress and cause mitochondrial dysfunction in microglia [62]. These neuroinflammatory responses and impaired redox processes are believed to be significant factors in the progression of neurological effects associated with prolonged COVID-19 [63]. In addition to inflammation, hypoxia plays a significant role in the pathogenesis of post-COVID-19 conditions. Systemic hypoxia arises from lung impairment, and patients with persistent lung issues often require supplemental oxygen. Studies have shown a correlation between cognitive impairment and the degree of oxygen supplementation required to alleviate respiratory challenges [64]. Furthermore, COVID-19 can induce organ-related ischemia by causing endothelial damage and hypercoagulation, thereby increasing the risk of vascular dysfunction [65]. The potential mechanisms underlying brain fog symptoms are shown in Fig. 3.
Based on our review, the possible interventions for brain fog include rehabilitation, noninvasive brain stimulation, and hyperbaric oxygen therapy. However, determining the most suitable intervention for individual patients is challenging. Due to the use of different outcome measures in the studies, we could not determine which intervention showed the most success regarding the primary outcome, treating brain fog symptoms. Nevertheless, certain parameters, such as treatment duration, adverse effects, and secondary outcomes, should be considered when considering which intervention to use. Patients who prefer shorter intervention methods may benefit from noninvasive brain stimulation, which has shown the lowest number of sessions required and the shortest time to treat brain fog symptoms. Moreover, patients with neuropsychiatric manifestations will benefit because noninvasive brain stimulation is useful for treating neuropsychiatric symptoms such as depression. Hyperbaric oxygen therapy, despite requiring a greater number of sessions, offers benefits in improving tissue perfusion, making it beneficial for patients experiencing chronic fatigue due to long-term COVID-19 and for patients suffering from persistent lung function impairment. Rehabilitation appears to be the safest choice among the interventions, with no reported adverse effects, and a study suggested possible improvements in depression symptoms. However, further research is necessary to ascertain the efficacy of rehabilitation due to the mixed results observed. An important aspect to consider is the significance of adherence to the treatment regimen for achieving successful outcomes. Therefore, rehabilitation may be recommended for patients with high adherence who prefer interventions without potential adverse effects or who are apprehensive about noninvasive brain stimulation and hyperbaric chamber treatments. Due to the limited number of studies on pharmacological interventions, it is challenging to determine their effect on the treatment of brain fog. However, based on the available studies, PEA-LUT is a possible treatment for brain fog and has been shown to be effective in treating parosmia. Further research is needed to determine the optimal pharmacological intervention for brain fog symptoms in post-COVID-19 patients (Fig. 3).
Potential mechanism underlying brain-fog symptoms caused by COVID-19. When COVID-19 patients gain access to the central nervous system (CNS), various pathways are activated, including direct invasion of CNS cells, retrograde axonal transport, and penetration through the endothelial cells of the blood‒brain barrier. Once inside the CNS, COVID-19 can prompt microglia to release proinflammatory agents, leading to mitochondrial dysfunction and oxidative stress. This cascade of events can lead to neuroinflammation, demyelination, and neurodegeneration. Moreover, systemic hypoxia and organ-related ischemia also participate in pathogenesis
It is important to note that only one study combined rehabilitation with other interventions. Wysokiński et al. combined transcranial direct current stimulation with cognitive rehabilitation. They concluded that transcranial direct current stimulation combined with another therapeutic intervention (caused by suprathreshold stimuli) may serve as an inducer of neuroplasticity, thus amplifying the training effects of cognitive rehabilitation. This hypothesis is supported by a study by Rodella et al., who combined transcranial direct current stimulation with cognitive training in patients with mild cognitive impairment [66]. Further studies comparing the efficacy of transcranial direct current stimulation alone versus transcranial direct current stimulation combined with cognitive training are necessary to better understand their respective impacts on brain fog symptoms and cognitive function.
The strength of our study was that we performed a comprehensive search of a wide number of electronic databases. The limitations of this review are the limited number of studies included, high heterogeneity due to the use of different scoring methods, the inclusion of case reports and case series with very small sample sizes, and the lack of meta-regression analysis.
Conclusion
The importance of finding the right intervention for brain fog symptoms is an important task for physicians because of the reduced quality of life and difficulty returning to their normal occupations. Our review revealed that noninvasive brain stimulation and hyperbaric oxygen therapy show promising results in the treatment of brain fog symptoms caused by long-COVID, showcasing improved perfusion and cortical excitability. Furthermore, both rehabilitation strategies and PEA-LUT administration have been associated with improvements in symptoms of brain fog. Future studies should explore combinations of interventions and include longer follow-up periods to assess the long-term effects of these treatments.
References
Parums DV (2023) Editorial: A rapid global increase in COVID-19 is due to the emergence of the EG.5 (Eris) Subvariant of Omicron SARS-CoV-2. Med Sci Monit 29:e942244. https://doi.org/10.12659/MSM.942244
Huerne K, Filion KB, Grad R, Ernst P, Gershon AS, Eisenberg MJ (2023) Epidemiological and clinical perspectives of long COVID syndrome. Am J Med Open 9:100033. https://doi.org/10.1016/j.ajmo.2023.100033
Venkatesan P (2021) NICE guideline on long COVID. Lancet Respir Med 9(2):129. https://doi.org/10.1016/S2213-2600(21)00031-X
Mahase E (2021) Covid-19: One in three has neurological or psychiatric condition diagnosed after covid infection, study finds. BMJ 373:n908. https://doi.org/10.1136/bmj.n908
Premraj L, Kannapadi NV, Briggs J et al (2022) Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci 434:120162. https://doi.org/10.1016/j.jns.2022.120162
Aghajani Mir M (2023) Brain Fog: a Narrative Review of the Most Common Mysterious Cognitive Disorder in COVID-19. Mol Neurobiol. https://doi.org/10.1007/s12035-023-03715-y
Lam GY, Damant RW, Ferrara G et al (2023) Characterizing long-COVID brain fog: a retrospective cohort study. J Neurol 270(10):4640–4646. https://doi.org/10.1007/s00415-023-11913-w
Líška D, Liptaková E, Babičová A, Batalik L, Baňárová PS, Dobrodenková S (2022) What is the quality of life in patients with long COVID compared to a healthy control group? Front Public Health 10:975992. https://doi.org/10.3389/fpubh.2022.975992
Nordvig AS, Rajan M, Lau JD et al (2023) Brain fog in long COVID limits function and health status, independently of hospital severity and preexisting conditions. Front Neurol 14:1150096. https://doi.org/10.3389/fneur.2023.1150096
Chatys-Bogacka Z, Mazurkiewicz I, Slowik J et al (2022) Brain fog and quality of life at work in non-hospitalized patients after COVID-19. Int J Environ Res Public Health 19(19):12816. https://doi.org/10.3390/ijerph191912816
Bolattürk ÖF, Soylu AC (2023) Evaluation of cognitive, mental, and sleep patterns of post-acute COVID-19 patients and their correlation with thorax CT. Acta Neurol Belg 123(3):1089–1093. https://doi.org/10.1007/s13760-022-02001-3
Brown LA, Ballentine E, Zhu Y, McGinley EL, Pezzin L, Abramoff B (2022) The unique contribution of depression to cognitive impairment in Post-Acute Sequelae of SARS-CoV-2 infection. Brain Behav Immun Health 22:100460. https://doi.org/10.1016/j.bbih.2022.100460
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Chang CH, Chen SJ, Chen YC, Tsai HC (2023) A 30-year-old woman with an 8-week history of anxiety, depression, insomnia, and mild cognitive impairment following COVID-19 who responded to accelerated bilateral theta-burst transcranial magnetic stimulation over the prefrontal cortex. Am J Case Rep 24:e938732. https://doi.org/10.12659/AJCR.938732
Kupferschmitt A, Jöbges M, Randerath J, Hinterberger T, Loew TH, Köllner V (2023) Attention deficits and depressive symptoms improve differentially after rehabilitation of post-COVID condition - A prospective cohort study. J Psychosom Res 175:111540. https://doi.org/10.1016/j.jpsychores.2023.111540
Sasaki N, Yamatoku M, Tsuchida T, Sato H, Yamaguchi K (2023) Effect of repetitive transcranial magnetic stimulation on long coronavirus disease 2019 with fatigue and cognitive dysfunction. Prog Rehabil Med 8:20230004. https://doi.org/10.2490/prm.20230004
De Luca P, Camaioni A, Marra P et al (2022) Effect of ultra-micronized Palmitoylethanolamide and Luteolin on olfaction and memory in patients with long COVID: results of a longitudinal study. Cells 11(16):2552. https://doi.org/10.3390/cells11162552
Samper-Pardo M, León-Herrera S, Oliván-Blázquez B, Méndez-López F, Domínguez-García M, Sánchez-Recio R (2023) Effectiveness of a telerehabilitation intervention using ReCOVery APP of long COVID patients: a randomized, 3-month follow-up clinical trial. Sci Rep 13(1):7943. https://doi.org/10.1038/s41598-023-35058-y
Rabaiotti P, Ciracì C, Donelli D et al (2023) Effects of multidisciplinary rehabilitation enhanced with neuropsychological treatment on post-acute SARS-CoV-2 cognitive impairment (Brain Fog): An observational study. Brain Sci 13(5):791. https://doi.org/10.3390/brainsci13050791
Bhaiyat AM, Sasson E, Wang Z et al (2022) Hyperbaric oxygen treatment for long coronavirus disease-19: a case report. J Med Case Rep. 16(1):80. https://doi.org/10.1186/s13256-022-03287-w
Wysokiński A, Szczepocka E, Szczakowska A (2023) Improved cognitive performance, increased theta, alpha, beta and decreased delta powers after cognitive rehabilitation augmented with tDCS in a patient with post-COVID-19 cognitive impairment (brain-fog). Psychiatry Res Case Rep 2(2):100164. https://doi.org/10.1016/j.psycr.2023.100164. ISSN 2773-0212
Braga LW, Oliveira SB, Moreira AS et al (2023) Long COVID neuropsychological follow-up: Is cognitive rehabilitation relevant? NeuroRehabilitation 53(4):517–534. https://doi.org/10.3233/NRE-230212
Sabel BA, Zhou W, Huber F et al (2021) Non-invasive brain microcurrent stimulation therapy of long-COVID-19 reduces vascular dysregulation and improves visual and cognitive impairment. Restor Neurol Neurosci 39(6):393–408. https://doi.org/10.3233/RNN-211249
Duñabeitia JA, Mera F, Baro Ó, Jadad-Garcia T, Jadad AR (2023) Personalized computerized training for cognitive dysfunction after COVID-19: A before-and-after feasibility pilot study. Int J Environ Res Public Health 20(4):3100. https://doi.org/10.3390/ijerph20043100
Hausswirth C, Schmit C, Rougier Y, Coste A (2023) Positive Impacts of a four-week neuro-meditation program on cognitive function in post-acute sequelae of COVID-19 patients: A randomized controlled trial. Int J Environ Res Public Health 20(2):1361. https://doi.org/10.3390/ijerph20021361
Noda Y, Sato A, Shichi M et al (2023) Real world research on transcranial magnetic stimulation treatment strategies for neuropsychiatric symptoms with long-COVID in Japan. Asian J Psychiatr 81:103438. https://doi.org/10.1016/j.ajp.2022.103438
Zilberman-Itskovich S, Catalogna M, Sasson E et al (2022) Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep 12:11252. https://doi.org/10.1038/s41598-022-15565-0
Bowen R, Arany PR (2023) Use of either transcranial or whole-body photobiomodulation treatments improves COVID-19 brain fog. J Biophotonics 16(8):e202200391. https://doi.org/10.1002/jbio.202200391
Robbins T, Gonevski M, Clark C et al (2021) Hyperbaric oxygen therapy for the treatment of long COVID: early evaluation of a highly promising intervention. Clin Med (Lond) 21(6):e629–e632. https://doi.org/10.7861/clinmed.2021-0462
Cenacchi V, Furlanis G, Menichelli A, Lunardelli A, Pesavento V, Manganotti P (2024) Co-ultraPEALut in subjective cognitive impairment following SARS-CoV-2 infection: An exploratory retrospective study. Brain Sci 14(3):293. https://doi.org/10.3390/brainsci14030293
Clare L, Kudlicka A, Oyebode JR et al (2019) Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: A multicentre randomised controlled trial (the GREAT trial). Int J Geriatr Psychiatry 34(5):709–721. https://doi.org/10.1002/gps.5076
Kudlicka A, Martyr A, Bahar-Fuchs A, Sabates J, Woods B, Clare L (2023) Cognitive rehabilitation for people with mild to moderate dementia. Cochrane Database Syst Rev 6(6):CD013388. https://doi.org/10.1002/14651858.CD013388.pub2
Munger KC, Martinez AP, Hyland MH (2021) The impact of cognitive rehabilitation on quality of life in multiple sclerosis: A pilot study. Mult Scler J Exp Transl Clin 7(3):20552173211040240. https://doi.org/10.1177/20552173211040239
Mizrachi Y, Shahrabani S, Nachmani M, Hornik A (2020) Obstacles to using online health services among adults age 50 and up and the role of family support in overcoming them. Isr J Health Policy Res 9(1):42. https://doi.org/10.1186/s13584-020-00398-x
Nauta IM, Bertens D, Fasotti L et al (2023) Cognitive rehabilitation and mindfulness reduce cognitive complaints in multiple sclerosis (REMIND-MS): A randomized controlled trial. Mult Scler Relat Disord 71:104529. https://doi.org/10.1016/j.msard.2023.104529
Hill NT, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A (2017) Computerized Cognitive training in older adults with mild cognitive impairment or dementia: A systematic review and meta-analysis. Am J Psychiatry 174(4):329–340. https://doi.org/10.1176/appi.ajp.2016.16030360
Kesikburun S (2022) Non-invasive brain stimulation in rehabilitation. Turk J Phys Med Rehabil 68(1):1–8. https://doi.org/10.5606/tftrd.2022.10608
Boscutti A, De Figueiredo JM, Razouq D, Murphy N, Cho R, Selvaraj S (2023) Noninvasive brain stimulation techniques for treatment-resistant depression: Transcranial Magnetic stimulation and transcranial direct current stimulation. Psychiatr Clin North Am 46(2):307–329. https://doi.org/10.1016/j.psc.2023.02.005
Wang T, Guo Z, Du Y et al (2021) Effects of noninvasive brain stimulation (NIBS) on cognitive impairment in mild cognitive impairment and alzheimer disease: A meta-analysis. Alzheimer Dis Assoc Disord 35(3):278–288. https://doi.org/10.1097/WAD.0000000000000464
Newhouse A, Kritzer MD, Eryilmaz H et al (2022) Neurocircuitry hypothesis and clinical experience in treating neuropsychiatric symptoms of postacute sequelae of severe acute respiratory syndrome coronavirus 2. J Acad Consult Liaison Psychiatry 63(6):619–627. https://doi.org/10.1016/j.jaclp.2022.08.007
Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A (2023) Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 48(1):191–208. https://doi.org/10.1038/s41386-022-01453-8
Hashmi JT, Huang YY, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR (2010) Role of low-level laser therapy in neurorehabilitation. PM R 2(12 Suppl 2):S292–S305. https://doi.org/10.1016/j.pmrj.2010.10.013
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3(Pt 3)):633–639. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00633.x
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72(3):443–454. https://doi.org/10.1016/j.neuron.2011.10.008
Calvert JW, Cahill J, Zhang JH (2007) Hyperbaric oxygen and cerebral physiology. Neurol Res 29(2):132–141. https://doi.org/10.1179/016164107X174156
Alashram AR, Padua E, Romagnoli C, Annino G (2023) Hyperbaric oxygen therapy for cognitive impairments in patients with traumatic brain injury: A systematic review. Appl Neuropsychol Adult 30(5):602–613. https://doi.org/10.1080/23279095.2022.2041418
Marcinkowska AB, Mankowska ND, Kot J, Winklewski PJ (2022) Impact of hyperbaric oxygen therapy on cognitive functions: A systematic review. Neuropsychol Rev 32(1):99–126. https://doi.org/10.1007/s11065-021-09500-9
Chen J, Zhang F, Zhao L et al (2020) Hyperbaric oxygen ameliorates cognitive impairment in patients with Alzheimer’s disease and amnestic mild cognitive impairment. Alzheimers Dement (N Y) 6(1):e12030. https://doi.org/10.1002/trc2.12030
Senniappan K, Jeyabalan S, Rangappa P, Kanchi M (2020) Hyperbaric oxygen therapy: Can it be a novel supportive therapy in COVID-19? Indian J Anaesth 64(10):835–841. https://doi.org/10.4103/ija.IJA_613_20
Peña-Villalobos I, Casanova-Maldonado I, Lois P et al (2018) Hyperbaric oxygen increases stem cell proliferation, angiogenesis and wound-healing ability of WJ-MSCs in diabetic mice. Front Physiol 9:995. https://doi.org/10.3389/fphys.2018.00995
Zhou Z, Daugherty WP, Sun D et al (2007) Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J Neurosurg 106(4):687–694. https://doi.org/10.3171/jns.2007.106.4.687
Hadanny A, Bechor Y, Catalogna M et al (2018) Hyperbaric oxygen therapy can induce neuroplasticity and significant clinical improvement in patients suffering from fibromyalgia with a history of childhood sexual abuse-randomized controlled trial. Front Psychol 9:2495. https://doi.org/10.3389/fpsyg.2018.02495
Efrati S, Ben-Jacob E (2014) Reflections on the neurotherapeutic effects of hyperbaric oxygen. Expert Rev Neurother 14(3):233–236. https://doi.org/10.1586/14737175.2014.884928
Gottfried I, Schottlender N, Ashery U (2021) Hyperbaric oxygen treatment-from mechanisms to cognitive improvement. Biomolecules 11(10):1520. https://doi.org/10.3390/biom11101520
Raciti L, Arcadi FA, Calabrò RS (2022) Could palmitoylethanolamide be an effective treatment for long-COVID-19? hypothesis and insights in potential mechanisms of action and clinical applications. Innov Clin Neurosci 19(1–3):19–25
Sarnelli G, Gigli S, Capoccia E et al (2016) Palmitoylethanolamide exerts antiproliferative effect and downregulates VEGF signaling in Caco-2 human colon carcinoma cell line through a selective PPAR-α-dependent inhibition of Akt/mTOR pathway. Phytother Res 30(6):963–970. https://doi.org/10.1002/ptr.5601
D’Agostino G, Russo R, Avagliano C, Cristiano C, Meli R, Calignano A (2012) Palmitoylethanolamide protects against the amyloid-β25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37(7):1784–1792. https://doi.org/10.1038/npp.2012.25
Ho WS, Barrett DA, Randall MD (2008) “Entourage” effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol 155(6):837–846. https://doi.org/10.1038/bjp.2008.324
Cipriano M, Esposito G, Negro L et al (2015) Palmitoylethanolamide regulates production of pro-angiogenic mediators in a model of β amyloid-induced astrogliosis in vitro. CNS Neurol Disord Drug Targets 14(7):828–837. https://doi.org/10.2174/1871527314666150317224155
Crunfli F, Carregari VC, Veras FP et al (2022) Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A 119(35):e2200960119. https://doi.org/10.1073/pnas.2200960119
Theoharides TC, Cholevas C, Polyzoidis K, Politis A (2021) Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. BioFactors 47(2):232–241. https://doi.org/10.1002/biof.1726
Clough E, Inigo J, Chandra D et al (2021) Mitochondrial Dynamics in SARS-COV2 Spike Protein Treated Human Microglia: Implications for Neuro-COVID [published correction appears in J Neuroimmune Pharmacol. 2021 Dec 11;:]. J Neuroimmune Pharmacol 16(4):770–784. https://doi.org/10.1007/s11481-021-10015-6
Ercegovac M, Asanin M, Savic-Radojevic A et al (2022) Antioxidant genetic profile modifies probability of developing neurological sequelae in long-COVID. Antioxidants (Basel) 11(5):954. https://doi.org/10.3390/antiox11050954
Lopez-Leon S, Wegman-Ostrosky T, Perelman C et al (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 11(1):16144. https://doi.org/10.1038/s41598-021-95565-8
Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM (2021) Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet 398(10300):599–607. https://doi.org/10.1016/S0140-6736(21)00896-5
Rodella C, Bernini S, Panzarasa S et al (2022) A double-blind randomized controlled trial combining cognitive training (CoRe) and neurostimulation (tDCS) in the early stages of cognitive impairment. Aging Clin Exp Res 34(1):73–83. https://doi.org/10.1007/s40520-021-01912-0
Funding
Open access funding provided by Bar-Ilan University. This work did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, AG, TL, YS; Methodology, AG, SS, LL, TL,; Formal Analysis, AG, YS; Data Curation, AG, TL, SS, LL; Writing – Original Draft Preparation, AG; Writing – Review & Editing, AG, YS, LL; Supervision, YS.
Corresponding author
Ethics declarations
Ethical compliance
Consent from the ethics committee was not required for the research.
Ethical approval
Ethical approval Consent from the ethics committee was not required for the research.
Informed consent
Informed consent Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gorenshtein, A., Liba, T., Leibovitch, L. et al. Intervention modalities for brain fog caused by long-COVID: systematic review of the literature. Neurol Sci 45, 2951–2968 (2024). https://doi.org/10.1007/s10072-024-07566-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-024-07566-w