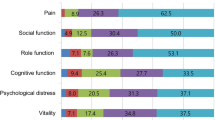
Abstract
Aims
Investigate if different clinical and psychophysical bedside tools can differentiate between district migraine phenotypes in ictal/perictal (cohort 1) and interictal (cohort 2) phases.
Method
This observational study included two independent samples in which patients were subgrouped into distinct clusters using standardized bedside assessment tools (headache frequency, disability, cervical active range of motion, pressure pain threshold in different areas): (A) cohort 1—ictal/perictal migraine patients were subgrouped, based on previous studies, into two clusters, i.e., Cluster-1.1 No Psychophysical Impairments (NPI) and Cluster-1.2 Increased Pain Sensitivity and Cervical Musculoskeletal Dysfunction (IPS-CMD); (B) cohort 2—interictal migraine patients were subgrouped into three clusters, i.e., Cluster-2.1 NPI, Cluster-2.2 IPS, and Cluster-2.3 IPS-CMD. Clinical characteristics (multiple questionnaires), somatosensory function (comprehensive quantitative sensory testing (QST)), and cervical musculoskeletal impairments (cervical musculoskeletal assessment) were assessed and compared across headache clusters and a group of 56 healthy controls matched for sex and age.
Results
Cohort 1: A total of 156 subjects were included. Cluster-1.2 (IPS-CMD) had higher headache intensity (p = 0.048), worse headache-related (p = 0.003) and neck-related disability (p = 0.005), worse quality of life (p = 0.003), and higher symptoms related to sensitization (p = 0.001) and psychological burden (p = 0.005) vs. Cluster-1.1(NPI). Furthermore, Cluster-1.2 (IPS-CMD) had (1) reduced cervical active and passive range of motion (p < 0.023), reduced functionality of deep cervical flexors (p < 0.001), and reduced values in all QST(p < 0.001) vs. controls, and (2) reduced active mobility in flexion, left/right lateral flexion (p < 0.045), and reduced values in QST (p < 0.001) vs. Cluster-1.1 (NPI). Cohort 2: A total of 154 subjects were included. Cluster-2.3 (IPS-CMD) had (1) longer disease duration (p = 0.006), higher headache frequency (p = 0.006), disability (p < 0.001), and psychological burden (p = 0.027) vs. Cluster-2.2 (IPS) and (2) higher headache-related disability (p = 0.010), neck-related disability (p = 0.009), and higher symptoms of sensitization (p = 0.018) vs. Cluster-2.1 (NPI). Cluster-2.3(IPS-CMD) had reduced cervical active and passive range of motion (p < 0.034), and reduced functionality of deep cervical flexors (p < 0.001), vs. controls, Custer-2.1 (NPI), and Cluster-2.2 (IPS). Cluster-2.2 (IPS) and 2.3 (IPS-CMD) had reduced QST values vs. controls (p < 0.001) and Cluster-2.1 (p < 0.039).
Conclusion
A battery of patient-related outcome measures (PROMs) and quantitative bedside tools can separate migraine clusters with different clinical characteristics, somatosensory functions, and cervical musculoskeletal impairments. This confirms the existence of distinct migraine phenotypes and emphasizes the importance of migraine phases of which the characteristics are assessed. This may have implications for responders and non-responders to anti-migraine medications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a common neurovascular brain disorder considered among the primary causes of disability worldwide [1], and no significant improvements were made in its burden between 1990 and 2016 [1, 2]. Thus, in recent years, researchers put great effort into developing a new class of drugs specifically targeting the CGRP peptide or receptors that could reduce the migraine burden [3]. However, this new therapy is known not to be efficient in all migraine patients [4]. Moreover, due to its high cost [5], this new class of drugs are not considered the first treatment approach in migraine management [6]. To enhance the cost-effectiveness of migraine treatment and reduce the burden of this disease, precision medicine principles should be applied clinically in migraine patients [7]. Precision medicine requires the use of various biomarkers to identify distinct phenotypes within the same medical condition with the aim to identify responders and non-responders to different treatment approaches [8]. In migraine patients, psychophysical characteristics is one parameter to be used to profile patients in different subgroups and predict treatment response [9,10,11,12]. The biomarkers used to profile migraine patients often involve time-consuming assessment that requires expensive tools [9,10,11,12], not always available or applicable in a clinical setting. We have recently applied fourteen clinical patient–related outcome measures (PROMs) and psychophysical bedside tools used in a clinical setting to profile and identify subgroups of migraine patients [13]. Episodic and chronic migraine patients were assessed in the ictal/perictal phase and interictal phase. In the ictal/perictal phase, two distinct clusters were identified, with one group showing no psychophysical impairment, and one showing increased pain sensitivity and cervical musculoskeletal dysfunctions, as well as higher disability. In the interictal phase, three distinct clusters could be identified, with one group showing no psychophysical impairment, one increased pain sensitivity, and one increased pain sensitivity and cervical musculoskeletal dysfunctions, as well as higher headache frequency, and disability [13].
However, as a limited number of clinical and psychophysical bedside tools were used to identify these distinct subgroups, the clinical validity should be further investigated before assuming these subgroups could be considered clinically different migraine phenotypes. The aim of this paper was to investigate the clinical importance of the distinct migraine subgroups by (1) assessing differences in clinical characteristics, including psychological burden, using a core set of PROMs and (2) assessing quantitative differences in somatosensory function and cervical musculoskeletal impairments using comprehensive quantitative sensory testing and cervical musculoskeletal assessment. These paper results will add important information to our previous finding [13] as the distinct migraine subgroups will be also compared to a control group of healthy subjects, and multiple clinical, psychological, and psychophysical characteristics will be assessed. The assessment of migraineur illness perception and psychological aspects using variously subjective measures (PROMs) together with objective measures of migraine psychophysical manifestation (quantitative sensory testing and cervical musculoskeletal assessment) will give important insights regarding the interaction between objective signs and subjective experience of the disease [14]. These results will help clinicians to tailor a more personalized treatment approach following the biopsychosocial model [14].
Method
Design
This multicenter, cross-sectional, observational study was conducted at the Parma and Genova Headache Center and approved by the Ligurian (244/2018) and “Area Vasta Emilia-Nord” (18,305/2019) regional ethic committee. All subjects signed an informed consent form and were assessed between April 2019 and February 2022. This study was based on two cohorts of migraine patients that have previously been the basis for a recent paper [13].
Population
Patients on waiting lists to receive their first visit to the Headache Center were invited to participate in this study. Men and women aged between 18 and 65 with episodic (EM) or chronic (CM) migraine with or without aura for at least 3 months were included. As psychophysical characteristics vary according to the migraine phase [15, 16], patients were divided into two distinct cohorts according to the migraine phase in which the psychophysical examination was performed.
In cohort 1, ictal/perictal EM and CM were included. EM patients were considered in the ictal phase if they had a headache during the visit and in the perictal phase if they have a headache within the 24 h before or after the visit [17, 18]. CM patients were considered in the ictal phase if they had any type of headache (with tension-type or migraine characteristics) during the visit. Patients were excluded if they had any other primary/secondary headache; less than 1 headache attack in 4 weeks; changes of headache characteristics, or onset of a “new” headache after COVID-19 infection/vaccination; any other neurologic, psychiatric, rheumatologic, or systemic pathology with medical diagnosis; history of head/neck trauma in the previous year; received cervical/head surgery; received manual therapy in the cervical spine, or cervical anesthetic block, or botulin injection in the last 6 months; changed the prophylactic treatment in the last 3 months; or were unable to speak and understand Italian.
In cohort 2, interictal EM and CM were included. EM patients were considered in the interictal phase if they were headache-free during the visit and did not have a headache within the 24 h before or after the visit [17, 18]. CM patients were considered in the interictal phase if they were headache-free during the visit. The exclusion criteria were the same used for ictal/perictal patients (cohort 1) with the exception that patients who used acute pharmacologic treatment in the 24 h before the assessment were excluded.
Control participants were recruited specifically for this study. They were defined as healthy subjects with a maximum of two headache episodes per year that did not fulfill the criteria for migraine or any other primary headache type with no family history of migraine or other primary headaches. The exclusion criteria for the control subjects were the same as the criteria used for migraine patients in cohort 1.
Procedure
The first screening was made by telephone interview in which patients were excluded if they presented any signs of red flags [19], or any exclusion criteria. Healthy controls were recruited from university students, hospital staff and university staff, and the general population through print and social media advertising. Then, a physical examination was performed in which one physiotherapist for each recruitment center (S.D., M.C.), blinded to the presence of headache, performed the assessment (psychophysical examination, questionnaire, and explanation of how to fulfill a diary for the following 4 weeks) and recorded the interval between the assessment and the last headache attack. Four weeks following the first evaluation, patients were visited by a neurologist who performed a diagnosis of headache according to the ICHD-3 [20]‚ and CM and EM patients with or without aura were included and divided into two cohorts that had undergone two separate analyses according to our previous suggestion [13].
Cohort 1: EM and CM in the ictal/perictal phase; cohort 2: EM and CM in the interictal phase. In each cohort, migraine patients were profiled into different clusters according to clinical and psychophysical characteristics [13]:
Cohort 1:
-
Cluster-1.1: Migraine with no psychophysical impairments (NPI)
-
Cluster-1.2: Migraine with increased pain sensitivity and cervical musculoskeletal dysfunctions (IPS-CMD)
Cohort 2:
-
Cluster-2.1: Migraine with no psychophysical impairments (NPI)
-
Cluster-2.2: Migraine with increase pain sensitivity (IPS)
-
Cluster-2.3: Migraine with increased pain sensitivity and cervical musculoskeletal dysfunctions (IPS-CMD)
Assessments
For each subject, general characteristics were assessed (Table 1). Migraine patients used a daily updated diary recording the total use of drugs and the frequency, intensity, and duration of headache attacks. Moreover, the headache side and total years lived with the headache were recorded (Table 2).
Questionnaires
-
Headache disability index (HDI): The HDI questionnaire was used to assess the emotional (HDI-E 0–52) and the physical (HDI-P 0–48) headache-related disability. The higher the score, the higher the headache-related disability [21].
-
Neck disability index (NDI): Neck-related disability was assessed using the NDI (0–100%), and the total score was calculated [22]. Then the subscale regarding physical function (personal care, lifting, work, driving, sleeping, and recreation; NDI-physical 0–100%) and mental function (neck pain, reading, headaches, and concentration; NDI-mental 0–100%) were calculated [23]. The higher the score, the higher the neck-related disability. NDI questionnaires were also used to identify patients with or without the presence of neck pain [16, 24].
-
Medical Outcomes Study Short Form 36 (SF-36): The SF-36 questionnaire was used to assess two components of quality of life: the physical health dimension (SF-36 physical, total score 0–100) and the mental health dimension (SF-36 emotional, total score 0–100). The lower the score, the lower the quality of life [25].
-
Central sensitization inventory (CSI): The CSI questionnaire was used to assess symptoms related to sensitization. The higher the score, the more symptoms related to central sensitization are present (0–100). A cutoff value of 40 is normally used in the literature [26].
-
Hospital Anxiety and Depression Scale (HADS): The HADS was used to assess the impact of anxiety (HADS-A) and depressive (HADS-D) symptoms. A higher score indicates a higher level of anxiety and/or depression (HADS: 0–21; HADS-D: 0–21) [27].
Psychophysical characteristics: cervical musculoskeletal impairments
Physical examination tests were used to assess the presence of cervical musculoskeletal impairments and were differentiated into tests aimed to evaluate cervical musculoskeletal functionality (cervical musculoskeletal dysfunction) and test aimed to identify the presence of referred pain [16].
Cervical musculoskeletal dysfunctions
-
Active range of motion (AROM): Cervical AROM (extension, flexion, left/right lateral flexion, left/right rotation) was recorded in degrees of movement with the cervical range of motion (CROM) device [28].
-
Flexion rotation test (FRT): Passive mobility of the upper cervical spine was assessed in degree with FRT. The subject was supine on the couch, and the therapist passively moved his neck to end-range cervical flexion. In this flexed position, the head and neck were passively rotated as far as possible within comfortable limits to the left and then to the right. The total range of motion during the FRT (sum left and right, °) was calculated in degrees using the CROM device [29].
-
Craniocervical flexion test (CCFT): The craniocervical flexion test assessed the function of deep cervical flexors muscles. A pressure biofeedback unit 20–30 mmHg was used. Subjects performed craniocervical flexion in five incremental stages (one stage every 2 mmHg), and the mmHg value that was held for 10 s without compensation was recorded as the activation pressure score (APS) [30].
Referred pain
-
Passive accessory intervertebral movements (PAIVMs): The number of positive vertebral segments (pain referred to the head region in control, typical migraine pain reproduction in patients) was assessed with PAIVMs over to C-0/C-1 and C-2/C-3 vertebral segments bilaterally [31].
-
Myofascial trigger points (MTrPs): MTrPs were defined as a hypersensitive spot in a taut band in the muscle belly that resulted in a referred pain, either recognized by the patient as the usual headache (active MTrPs) or referred into a non-headache region (latent MTrPs). The presence of MTrPs was assessed bilaterally in the temporal muscles, masseter muscle, sternocleidomastoid muscle, suboccipital muscles, splenius muscles, and trapezius muscle. The total number of active and latent trigger points was recorded [32].
Psychophysical characteristics: somatosensory function
Quantitative sensory testing (QST) was performed from distal pain-free areas first, then the cervical area, and finally the trigeminal area (symptomatic side in patients with unilateral migraine; dominant side in patients with side/shift or bilateral migraine and in controls) to assess signs related to sensitization [15]:
-
Static pressure pain threshold (sPPT): sPPT to hand-held electronic algometry (Somedic-AB, Sweden), probe area 1cm2, 30 kPa/s force increase)) [15] was assessed over the trigeminal area (temporalis muscle), upper cervical spine (left and right articular pillars of C1 and C2 vertebral segments), lower cervical spine (left and right articular pillars of C4 and C6 vertebral segments); distal pain-free areas (second metacarpophalangeal joint of the dominant hand; tibialis anterior muscle of the dominant leg).
-
Dynamic pressure pain threshold (dPPT): dPPT to a dynamic algometry (constant force spring controlled from 550 to 5300 g) was assessed over the posterior aspect of the neck (left and right sides) [15].
-
Mechanical pain threshold (MPT): MPT to pinprick stimulation (from 0.80 to 50.1 g metal probes) was assessed over the trigeminal area (temporalis muscle) and distal pain-free areas (thenar eminence of the dominant hand) [15].
For sPPT, dPPT, and MPT, the lower the threshold, the more sensitization:
-
• Wind-up ratio (WUR): The WUR was calculated to assess the temporal summation of mechanical pain. The patient was given a pain rating (11-point numeric rating scale) for the first and last stimulus of 10 stimuli given with pinprick over temporalis (50.1 g). The difference between the pain rating of the last of a ten stimuli series and the first stimulus was calculated (WUR) [15]. A positive WUR was a sign of increased temporal summation of pain, and the higher the WUR, the more the sensitization.
Details of the assessment were previously presented [15, 16, 33].
Statistical analysis
The sample size was calculated using G*Power 3.1. To achieve a moderate/large effect size (f: 0.35) with a power of 85% and an alpha level of 0.05 with a general linear model using 4 covariates and 3 groups (cohort 1), 131 subjects were required. In cohort 2, where 4 covariates and 4 groups were used, 139 subjects were required [34].
Differences across migraine groups in clinical characters were investigated with the t-test, Mann–Whitney test, or the chi-square test according to variable type and distribution when two migraine clusters were present. When more than two clusters were present, differences in general characters and frequency were investigated with the ANOVA, Kruskal–Wallis test, or chi-square test, according to variable type and distribution. The Bonferroni, the Mann–Whitney test, and the chi-square test were used to run post hoc analyses respectively for ANOVA, the Kruskal–Wallis test, and the chi-square test using a Bonferroni corrected p-value.
Differences in psychophysical characteristics across migraine groups and controls were investigated by transforming non-normal distributed variables to fulfill the normality assumption (the normality of the data was assessed with the Shapiro–Wilk test). The same sample of healthy controls was used as comparison both in cohort 1 and in cohort 2 analysis. To avoid that differences in general characteristics could bias the results, a general linear model was performed, including age, gender, body mass index, and use of acute pharmacological treatment in the 24 h before the evaluation as covariates in the models. A Bonferroni-adjusted post hoc analysis was performed to make single-group comparisons when more than two clusters were compared. Subjects with any missing data were excluded from the analysis in which data were not available. The threshold accepted for the statistical significance of the results was p < 0.05, and tests of statistical significance were two-tailed. Statistical analyses were performed using the SPSS software (version 24).
Results
A total of 779 subjects were initially screened. A total of 56 healthy subjects, 100 migraine patients assessed in the ictal/perictal phase (cohort 1), and 98 migraine patients assessed in the interictal phase (cohort 2) were included (Fig. 1). The general characteristics of the population included are reported in Table 1.
Cohort 1: ictal/perictal migraine patients
Clinical characteristics
Compared to Cluster-1.1 (NPI), Cluster-1.2 (IPS-CMD) had higher headache intensity (p = 0.048), worse HDI-E (p = 0.003), NDI (p = 0.005), NDI-physical (p = 0.005), NDI-mental (p = 0.023), SF-36 physical (p = 0.003), CSI (p = 0.001), HADS-A (p = 0.005), HADS-D (p = 0.005), and higher percentage of patients with neck pain (p = 0.012). No other differences were observed (Table 2a).
Psychophysical characteristics
Cluster-1.2 (IPS-CMD) had the following:
-
Compared to controls: reduced AROM in flexion, extension, right and left lateral flexion, right and left rotation, FRT, APS, and higher number of MTrPs and PAIVMs (all, p < 0.023). Higher trigeminal WUR (p = 0.019), lower trigeminal MPT (5.7(7.3) g vs 22.1(18.5) g) and sPPT, sPPT over upper and lower cervical spine, cervical dPPT, hand sPPT and MPT, and leg sPPT (all, p < 0.001).
-
Compared to Cluster-1.1 (NPI): reduced AROM in flexion, right and left lateral flexion, and higher number of MTrPs and PAIVMs (all, p = 0.045). Lower trigeminal, upper and lower cervical sPPT, cervical dPPT, hand and leg sPPTs (all, p < 0.001).
Cluster-1.1 (NPI) had compared to controls the following:
-
• Reduced APS and higher number of MTrPs and PAIVMs (all, p < 0.001). Higher trigeminal WUR (p = 0.028) (higher sensitization) and higher sPPT over temporalis, upper cervical spine, and leg (lower sensitization) (all, p < 0.049).
No other differences were observed (Table 3a).
Cohort 2: interictal migraine patients
Clinical characteristics
Cluster-2.3 (IPS-CMD) had the following:
-
Compared to Cluster-2.1 (NPI): worse HDI-E (p = 0.010), NDI-physical (p = 0.009), and CSI (p = 0.018).
-
Compared to Cluster-2.2 (IPS): longer disease duration (p = 0.006), higher headache frequency (p = 0.006), more use of drugs (p = 0.009), worse HDI-P (p = 0.003), HDI-E (p < 0.001), and HADS-D (p = 0.027)
No other differences were observed (Table 2b).
Psychophysical characteristics
Cluster-2.3 (IPS-CMD) had the following:
-
Compared to controls: reduced AROM in flexion, extension, right and left lateral flexion, right and left rotation, FRT, APS, and higher number of MTrPs and PAIVMs (all, p < 0.001). Lower trigeminal MPT and sPPT, sPPT over upper and lower cervical spine, cervical dPPT, hand sPPT and MPT (all, p < 0.023).
-
Compared to Cluster-2.1 (NPI): reduced AROM in flexion, right and left lateral flexion, right and left rotation, FRT, and APS (all, p < 0.034). Lower trigeminal sPPT, sPPT over upper and lower cervical spine, hand and leg sPPTs (all, p < 0.001).
-
Compared to Cluster-2.2 (IPS): reduced AROM in flexion, extension, right and left lateral flexion, right and left rotation, FRT, APS (all, p < 0.001).
Cluster-2.2 (IPS) had the following:
-
Compared to controls: reduced FRT, APS, and higher number of MTrPs and PAIVMs (all, p < 0.001). Lower trigeminal MPT and sPPT, sPPT over upper and lower cervical spine, cervical dPPT, hand sPPT and MPT (all, p < 0.015).
-
Compared to Cluster-2.1 (NPI): lower trigeminal sPPT, sPPT over upper and lower cervical spine, cervical dPPT, hand and leg sPPTs (all, p < 0.039).
Cluster-2.1 (NPI) had compared to controls the following:
-
• Reduced FRT, APS, and higher number of MTrPs and PAIVMs (all, p < 0.006). Lower trigeminal MPT (higher sensitization) and higher hand and leg sPPTs (lower sensitization) (all, p < 0.014).
Discussion
This paper results confirmed the existence of distinct migraine subgroups and associated phenotyping based on fourteen clinical PROMs and psychophysical bedside tools performed at different phases of the migraine cycle. These distinct migraine phenotypes showed significant differences in clinical presentations, somatosensory functions, and cervical musculoskeletal impairments, and further clinical importance is to be explored. As migraine patients with worse clinical and psychophysical characteristics also had worse psychological burden, a biopsychosocial approach should be considered in these migraine patients [14].
Moreover, these results added important information to what was found in a study just published by our research group [13]. First, even if most migraine patients (80%) showed increased pain sensitivity, 20% of them had reduced pain sensitivity (hypoalgesia) compared to healthy controls. Secondly, facilitation of the trigeminal temporal summation of pain seems to be a characteristic peculiar to ictal migraine patients, independent of the presence of increased pain sensitivity [15]. Third, subtle cervical musculoskeletal impairments seem to be present in all migraine subgroups compared to healthy subjects, strengthening the link existing between these physical characteristics and migraine pathophysiology. Fourth, even with different frequencies, all migraine subgroups had neck pain, suggesting that distinct mechanisms could underlie neck pain in the migraine population. In patients with no increased pain sensitivity but subtle cervical musculoskeletal impairments, neck pain is likely driven by peripheral mechanisms. On the other hand, in patients with increased pain sensitivity and cervical musculoskeletal impairments, both peripheral and central mechanisms could exist. Finally, in interictal migraine patients, impaired cervical musculoskeletal functionality, more than increased pain sensitivity, seems to be correlated with worse clinical characteristics and physiological burden.
Migraine subgroups during the ictal/perictal phase
Clinical characteristics
Migraine patients with perictal/ictal increased pain sensitivity and cervical musculoskeletal dysfunctions (Cluster-1.2, IPS-CMD) had worse clinical characteristics compared to patients with no psychophysical impairment (Cluster-1.1, NPI) [35,36,37] confirming that this subgroup is worse affected by the disease. Cluster-1.2 (IPS-CMD) had higher headache intensity and disability, worse neck-related disability, worse quality of life, higher symptoms related to sensitization, and worse psychological burden. Due to the study design, it was not possible to identify which relationship exists between increased pain sensitivity, cervical musculoskeletal impairments, worse clinical characteristics, and psychological burden. On one hand, the enhanced pain sensitivity and the reduction in cervical musculoskeletal functionality occurring during the migraine attack [15, 16, 38] could lead to higher headache intensity [39, 40]. This will, in turn, cause higher disability, worse quality of life, and higher psychological burden [37, 41, 42]. On the other hand, individual psychological and social factors can influence patients’ elaboration of the pain experience leading to a worse perception of the disease, increased pain sensitivity, higher headache intensity, and worse disability [14]. This attitude regarding the pain experience will, in turn, cause an avoidance behavior during the headache attack, impairing cervical musculoskeletal functionality [43]. Future longitudinal studies should assess the relationship existing between increased pain sensitivity, cervical musculoskeletal impairments, worse clinical characteristics, and psychological burden in migraine patients.
Psychophysical characteristics
Cervical musculoskeletal impairments
This study results showed, using a comprehensive cervical musculoskeletal evaluation, that Cluster-1.2 (IPS-CMD) had worse cervical musculoskeletal impairments compared to Cluster-1.1 (NPI) and healthy controls. As Cluster-1.2 also had increased widespread pain sensitivity, a relationship between musculoskeletal impairments and pain sensitivity seems to occur in migraine patients in proximity and during a headache attack [44, 45]. Even if patients in Cluster-1.2 (NPI) had no reduction of cervical active and passive mobility, they showed worse functionality of deep cervical flexor muscles and a higher number of myofascial and articular areas that can reproduce referred pain compared to healthy subjects. The reduction in deep cervical flexor muscle functionality was observed in both migraine subgroups compared to controls, without difference between Cluster-1.1 (NPI) and Cluster-1.2 (IPS-CMD). These results support that the craniocervical flexion test could differentiate migraine patients compared to healthy subjects but did not seem to have a direct relationship with worse clinical characteristics in migraine patients [46]. On the other hand, Cluster-1.1 (NPI) had a higher number of myofascial and articular areas that can reproduce referred pain compared to controls, but lower compared to Cluster-1.2 (IPS-CMD). Thus, due to their higher prevalence, an increased number of Myofascial TrPs and positive cervical vertebral segments could be a characteristic common to most migraine patients [31, 47]. However, they seem to be more enhanced in Cluster-1.2 (IPS-CMD), suggesting a relationship with worse clinical characteristics in migraine patients [16].
Somatosensory function
Using a comprehensive quantitative sensory testing protocol, the data confirmed increased trigeminal, cervical, and widespread pain sensitivity during the migraine attack occurred in 81% of the migraine population (IPS-CMD) [38]. The remaining 19% of patients (NPI) showed higher QST value (reduced pain sensitivity) compared to controls, suggesting that the increased pain sensitivity that characterized the migraine attack [38, 48] did not occur in all migraineurs, with a subgroup of patients showing signs of reduced pain sensitivity [49].
However, temporal summation of pain in the trigeminal area was facilitated in both migraine clusters and was peculiar to the ictal phase [15]. As WUR was increased also in patients with reduced pain sensitivity, different mechanisms could underlie the facilitation in temporal summation of pain and the increase in pain sensitivity [50, 51].
Increased trigeminal, cervical, and widespread pain sensitivity could be a sign of pain amplification in the trigeminocervical complex and subcortical/cortical brain areas [38, 52]. On the other hand, the facilitation of trigeminal temporal summation of pinprick pain could reflect enhanced gain in the trigeminocervical complex [52]. Both presynaptic and postsynaptic sensitization mechanisms and impairment of descending pain modulation [53] mechanisms could facilitate the trigeminal temporal summation of pain [54]. As WUR was also facilitated in migraine patients with reduced pain sensitivity in the trigeminocervical area, who did not show signs of enhanced pain amplification at the trigeminocervical level, it is more likely that impairment of descending pain modulation mechanisms could account for the facilitation of trigeminal temporal summation of pain observed [53]. Future studies should assess the impairment of descending pain modulation mechanisms in migraine patients focusing on different phenotypes and migraine phases.
Migraine subgroups during the interictal phase
Clinical characteristics
The migraine subgroup with interictal increased pain sensitivity and cervical musculoskeletal dysfunctions (Cluster-2.3, IPS-CMD) was worse affected by the disease, with worse headache and neck-related disability and higher symptoms of sensitization compared to patients with no psychophysical impairment (Cluster-2.1, NPI), and worse headache characteristics, disability, and psychological burden compared to patients with increased pain sensitivity (Cluster-2.2, IPS).
In a previous paper, we hypothesize that, as they share similar prevalence, general, and clinical characteristics, Cluster-1.1 (NPI, ictal) and Cluster-2.1 (NPI interictal) could represent the same subgroup assessed in different migraine phases [55]. On the other hand, the remaining 80% of the sample will be represented by one group when patients were assessed ictally/preictally (Cluster-1.2 IPS-CMD), and by two different subgroups when patients were assessed interictally (Cluster-2.3 IPS-CMD and Cluster-2.2 IPS interictally). As Cluster-2.3 (IPS-CMD) had longer disease duration and higher headache frequency compared to Cluster-2.2 (IPS), these clusters could be considered a clinical continuum representing the two extremities in terms of disease progression of a single group assessed ictally/preictally (Cluster-1.2 IPS-CMD). This paper’s results seem to confirm this hypothesis using multiple questionnaires to assess clinical characteristics [55].
Interestingly, Cluster-2.3 (IPS-CMD) but not Cluster-2.2 (IPS), had worse neck-rerated disability compared to Cluster-2.1 (NPI). Moreover, even if it did not reach a statistical significance, Cluster-2.3 (IPS-CMD) had worse neck-related disability also compared to Cluster-2.2 (IPS). As Cluster-2.3 (IPS-CMD) and Cluster-2.2 (IPS) had signs of increased pain sensitivity, but only the former also had cervical musculoskeletal dysfunctions, not only “central sensitization” mechanisms [23, 56], but also peripheral mechanisms [57, 58], could influence the presence and the burden of neck pain in migraine patients.
Psychophysical characteristics
Cervical musculoskeletal impairments
When migraine patients were assessed in the interictal phase, one migraine subgroup (Cluster-2.3, IPS-CMD) had worse cervical musculoskeletal impairments with reduced active and passive cervical mobility and worse functionality of deep cervical flexor muscles compared to matched healthy controls, to Cluster-2.1(NPI), and to Cluster-2.2(IPS). As Cluster-2.3(IPS-CMD) was the subgroup worse affected by the disease, a relationship seems to occur between cervical musculoskeletal functionality and disease burden [16, 59], outlining the importance to assess and address cervical musculoskeletal dysfunctions in migraine patients.
As reduced cervical passive mobility and deep cervical flexor muscles’ functionality, and higher areas that reproduce referred pain, were also observed in Cluster-2.1 (NPI) and Cluster-2.2 (IPS) compared to controls, subtle cervical musculoskeletal impairments could occur independently by the presence of increased pain sensitivity [56] and the clinical manifestation of the disease [46]. On the other hand, reduced cervical active range of motion seems to be peculiar to a subgroup with worse clinical characteristics [16, 59], increased pain sensitivity, and further impairment of cervical musculoskeletal functionality. Thus, to enhance the clinical validity of the cervical musculoskeletal assessment, clinicians should consider the degree of cervical musculoskeletal impairments. Cervical active mobility should be considered a key driver to evaluate the extent of cervical musculoskeletal impairments.
Somatosensory function
Cluster-2.2 (IPS) and 2.3 (IPS-CMD) had increased trigeminal, cervical, and widespread pain sensitivity assessed with multiple QST compared to Cluster-2.1 (NPI) and controls. On the other hand, like Cluster-1.1 (NPI preictal/ictal), Cluster-2.1 (NPI interictal) had reduced pain sensitivity compared to controls supporting that these two subgroups could represent the same cluster assessed in different migraine phases. Therefore, about 20% of migraine patients showed reduced pain sensitivity independently by the headache phase [49].
As Cluster-2.2 (IPS) and 2.3 (IPS-CMD) had a similar somatosensory profile to Cluster-1. 2(IPS), they could be considered two subgroups of the same cluster assessed in preictal/ictal phase (Cluster-1.2 IPS).
If activity-dependent sensitization mechanisms could account for the preictal/ictal increased pain sensitivity, late-onset transcription-dependent sensitization mechanisms seem to account for the interictal increased pain sensitivity [52]. The fact that, among the 80% of migraine patients with long-lasting increased pain sensitivity, only a subgroup of patients with a longer disease duration also had cervical musculoskeletal dysfunctions suggested that late-onset transcription-dependent sensitization mechanisms protracted over time could affect cervical motor behavior [44, 45]. However, future longitudinal studies should assess the bidirectional relationship between increased pain sensitivity and cervical musculoskeletal dysfunction in migraine patients.
Limitation
The population was recruited from specialized headache centers, and over two-third of the patients were excluded for age, concomitant pathologies, and concomitant diagnosis of other headache types. Thus, the external validity of these results should be interpreted with caution.
Development of pain sensitization is a time-dependent phenomenon that gradually develops approaching the migraine attack [17, 60]. Therefore, the inclusion of perictal and ictal migraine patients and the lack of controlling for the distance from the last/next headache attack performed in cohort 1 could have biased the results. However, in the previous paper in which different subgroups were defined, it was shown that the two clusters observed in cohort 1 did not differ in the headache phase or hours from the last/next headache attack [13].
Conclusion
Distinct migraine subgroups identified in different migraine phases by a set of clinical PROMs and quantitative bedside assessment tools could be considered clinically different migraine phenotypes. These distinct phenotypes showed different clinical and psychophysical characteristics assessed by multiple clinical-related questionnaires, comprehensive quantitative sensory testing, and cervical musculoskeletal assessment. Some specific parameters such as increased disability, lower cervical active range of motion, and reduced pressure pain thresholds were specifically sensitive to separate the distinct phenotypes. The clinical implications of the different phenotypes may provide different responses to migraine treatments and need further exploration.
References
Vos T, Abajobir AA, Abbafati C et al (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
Reuter U (2018) GBD 2016: still no improvement in the burden of migraine. Lancet Neurol 17(11):929–930. https://doi.org/10.1016/S1474-4422(18)30360-0
Ashina M, Buse DC, Ashina H et al (2021) Migraine: integrated approaches to clinical management and emerging treatments. Lancet 397(10283):1505–1518. https://doi.org/10.1016/S0140-6736(20)32342-4
Ashina M. Migraine. Ropper AH (2020) ed. New England Journal of Medicine. 383(19):1866-1876. https://doi.org/10.1056/NEJMra1915327
Sussman M, Benner J, Neumann P, Menzin J (2018) Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia 38(10):1644–1657. https://doi.org/10.1177/0333102418796842
Ailani J, Burch RC, Robbins MS (2021) The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache 61(7):1021–1039. https://doi.org/10.1111/head.14153
Ashina M, Terwindt GM, Al-Karagholi MAM et al (2021) Migraine: disease characterisation, biomarkers, and precision medicine. Lancet 397(10283):1496–1504. https://doi.org/10.1016/S0140-6736(20)32162-0
Edwards RR, Dworkin RH, Turk DC et al (2016) Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 157(9):1851–1871. https://doi.org/10.1097/j.pain.0000000000000602
Peng KP, Basedau H, Oppermann T, May A (2022) Trigeminal sensory modulatory effects of galcanezumab and clinical response prediction. Pain 163(11):2194–2199. https://doi.org/10.1097/j.pain.0000000000002614
Ashina S, Melo-Carrillo A, Szabo E, Borsook D, Burstein R (2023) Pre-treatment non-ictal cephalic allodynia identifies responders to prophylactic treatment of chronic and episodic migraine patients with galcanezumab: a prospective quantitative sensory testing study (NCT04271202). Cephalalgia 43(3):033310242211478. https://doi.org/10.1177/03331024221147881
Kisler LB, Weissman-Fogel I, Coghill RC, Sprecher E, Yarnitsky D, Granovsky Y (2019) Individualization of migraine prevention: a randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain 35(9):753–765. https://doi.org/10.1097/AJP.0000000000000739
Pan LLH, Wang YF, Ling YH et al (2022) Pain sensitivities predict prophylactic treatment outcomes of flunarizine in chronic migraine patients: a prospective study. Cephalalgia 42(9):899–909. https://doi.org/10.1177/03331024221080572
Di Antonio S, Arendt-Nielsen L, Ponzano M, et al. Profiling migraine patients according to clinical and psychophysical characteristics: a cluster analysis approach. Pain Medicine. Published online May 3, 2023. https://doi.org/10.1093/pm/pnad048
Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC (2007) The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 133(4):581–624. https://doi.org/10.1037/0033-2909.133.4.581
Di Antonio S, Castaldo M, Ponzano M et al (2022) Trigeminal and cervical sensitization during the four phases of the migraine cycle in patients with episodic migraine. Headache 62(2):176–190. https://doi.org/10.1111/head.14261
Di Antonio S, Arendt-Nielsen L, Ponzano M et al (2022) Cervical musculoskeletal impairments in the 4 phases of the migraine cycle in episodic migraine patients. Cephalalgia 42(9):827–845. https://doi.org/10.1177/03331024221082506
Sand T, Zhitniy N, Nilsen KB, Helde G, Hagen K, Stovner LJ (2008) Thermal pain thresholds are decreased in the migraine preattack phase. Eur J Neurol 15(11):1199–1205. https://doi.org/10.1111/j.1468-1331.2008.02276.x
Uglem M, Omland PM, Nilsen KB et al (2017) Does pain sensitivity change by migraine phase? A blinded longitudinal study. Cephalalgia 37(14):1337–1349. https://doi.org/10.1177/0333102416679955
Do TP, Remmers A, Schytz HW et al (2019) Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology 92(3):134–144. https://doi.org/10.1212/WNL.0000000000006697
Olesen J (2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211. https://doi.org/10.1177/0333102417738202
Jacobson GP, Ramadan NM, Norris L, Newman CW. Headache Disability Inventory (HDI): short-term test-retest reliability and spouse perceptions.
Monticone M, Ferrante S, Vernon H, Rocca B, Dal Farra F, Foti C (2012) Development of the Italian version of the neck disability index: cross-cultural adaptation, factor analysis, reliability, validity, and sensitivity to change. Spine (Phila Pa 1976). 37(17). https://doi.org/10.1097/BRS.0b013e3182579795
Liang Z, Thomas L, Jull G, Treleaven J (2022) the neck disability index reflects allodynia and headache disability but not cervical musculoskeletal dysfunction in migraine. Phys Ther 102(5). https://doi.org/10.1093/ptj/pzac027
Di Antonio S, Arendt-Nielsen L, Ponzano M, et al (2023) Migraine patients with and without neck pain: Differences in clinical characteristics, sensitization, musculoskeletal impairments, and psychological burden. Musculoskelet Sci Pract. Published online June:102800. https://doi.org/10.1016/j.msksp.2023.102800
McHorney CA, Ware JE, Raczek AE (1993) The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31(3):247–263. https://doi.org/10.1097/00005650-199303000-00006
Chiarotto A, Viti C, Sulli A, Cutolo M, Testa M, Piscitelli D (2018) Cross-cultural adaptation and validity of the Italian version of the Central Sensitization Inventory. Musculoskelet Sci Pract 37:20–28. https://doi.org/10.1016/j.msksp.2018.06.005
Costantini M, Musso M, Viterbori P et al (1999) Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer 7(3):121–127. https://doi.org/10.1007/s005200050241
Fletcher JP, Bandy WD (2008) Intrarater reliability of CROM measurement of cervical spine active range of motion in persons with and without neck pain. J Orthop Sports Phys Ther 38(10):640–645. https://doi.org/10.2519/jospt.2008.2680
Hall TM, Robinson KW, Fujinawa O, Akasaka K, Pyne EA (2008) Intertester reliability and diagnostic validity of the cervical flexion-rotation test. J Manipulative Physiol Ther 31(4):293–300. https://doi.org/10.1016/j.jmpt.2008.03.012
Jull GA, O’Leary SP, Falla DL (2008) Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipulative Physiol Ther 31(7):525–533. https://doi.org/10.1016/j.jmpt.2008.08.003
Watson DH, Drummond PD (2012) Head pain referral during examination of the neck in migraine and tension-type headache. Headache 52(8):1226–1235. https://doi.org/10.1111/j.1526-4610.2012.02169.x
Mayoral del Moral O, Torres Lacomba M, Russell IJ, Sánchez Méndez Ó, Sánchez Sánchez B (2018) Validity and reliability of clinical examination in the diagnosis of myofascial pain syndrome and myofascial trigger points in upper quarter muscles. Pain Med (United States) 19(10):2039–2050. https://doi.org/10.1093/pm/pnx315
Di Antonio S, Castaldo M, Ponzano M et al (2021) Disability, burden, and symptoms related to sensitization in migraine patients associate with headache frequency. Scand J Pain 21(4):766–777. https://doi.org/10.1515/sjpain-2021-0050
Fernández-De-Las-Peñas C, Ambite-Quesada S, Florencio LL, Palacios-Ceña M, Ordás-Bandera C, Arendt-Nielsen L (2019) Catechol-O-methyltransferase Val158Met polymorphism is associated with anxiety, depression, and widespread pressure pain sensitivity in women with chronic, but not episodic, migraine. Pain Med (United States) 20(7):1409–1417. https://doi.org/10.1093/pm/pny237
Palacios-Ceña M, Florencio LL, Ferracini GN et al (2016) Women with chronic and episodic migraine exhibit similar widespread pressure pain sensitivity. Pain Med (United States) 17(11):2127–2133. https://doi.org/10.1093/PM/PNW056
Palacios Ceña M, Castaldo M, Kelun W et al (2017) Widespread pressure pain hypersensitivity is similar in women with frequent episodic and chronic tension-type headache: a blinded case–control study. Headache 57(2):217–225. https://doi.org/10.1111/head.12982
Aguila MER, Rebbeck T, Pope A, Ng K, Leaver AM (2018) Six-month clinical course and factors associated with non-improvement in migraine and non-migraine headaches. Cephalalgia 38(10):1672–1686. https://doi.org/10.1177/0333102417744360
Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH (2000) An association between migraine and cutaneous allodynia. Ann Neurol 47(5):614–624. https://doi.org/10.1002/1531-8249(200005)47:5%3c614::AID-ANA9%3e3.0.CO;2-N
Arendt-Nielsen L, Nie H, Laursen MB et al (2010) Sensitization in patients with painful knee osteoarthritis. Pain 149(3):573–581. https://doi.org/10.1016/j.pain.2010.04.003
Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL (2012) Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 64(9):2907–2916. https://doi.org/10.1002/art.34466
Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA (2019) Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain 160(9):1920–1932. https://doi.org/10.1097/j.pain.0000000000001590
Polk AN, Protti TA, Smitherman TA (2020) Allodynia and disability in migraine: the mediating role of stress. Headache 60(10):2281–2290. https://doi.org/10.1111/head.14012
Jones LE, O’Shaughnessy DFP (2014) The pain and movement reasoning model: introduction to a simple tool for integrated pain assessment. Man Ther 19(3):270–276. https://doi.org/10.1016/j.math.2014.01.010
Jorge JG, Cabral ALC e. S, Moreira VMPS, Hattori WT, Dionisio VC (2021) Hyperalgesia affects muscle activity and knee range of motion during a single-limb mini squat in individuals with knee osteoarthritis: a cross-sectional study. BMC Musculoskelet Disord 22(1). https://doi.org/10.1186/s12891-021-03947-w
Arendt-Nielsen L, Sci M, Graven-Nielsen T (2008) Muscle pain: sensory implications and interaction with motor control. https://journals.lww.com/clinicalpain
Rodrigues A, Florencio LL, Martins J et al (2021) Craniocervical flexion test in patients with migraine: discriminative validity and accuracy. Int J Clin Pract 75(7). https://doi.org/10.1111/ijcp.14248
Luedtke K, Starke W, May A (2018) Musculoskeletal dysfunction in migraine patients. Cephalalgia 38(5):865–875. https://doi.org/10.1177/0333102417716934
Scholten-Peeters GGM, Coppieters MW, Durge TSC, Castien RF (2020) Fluctuations in local and widespread mechanical sensitivity throughout the migraine cycle: a prospective longitudinal study. J Headache Pain 21(1). https://doi.org/10.1186/s10194-020-1083-z
Pan LLH, Wang YF, Lai KL et al (2020) Mechanical punctate pain threshold is associated with headache frequency and phase in patients with migraine. Cephalalgia 40(9):990–997. https://doi.org/10.1177/0333102420925540
Greenspan J, Slade G, Rathnayaka N, Fillingim R, Ohrbach R, Maixner W (2020) Experimental pain sensitivity in subjects with temporomandibular disorders and multiple other chronic pain conditions: the OPPERA prospective cohort study. J Oral Facial Pain Headache 34:s43–s56. https://doi.org/10.11607/ofph.2583
Woolf CJ (1996) Editorial Windup and Central Sensitization Are Not Equivalent. Vol 66.
Ji RR, Kohno T, Moore KA, Woolf CJ (2003) Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci 26(12):696–705. https://doi.org/10.1016/j.tins.2003.09.017
Williams AE, Miller MM, Bartley EJ, McCabe KM, Kerr KL, Rhudy JL (2019) Impairment of inhibition of trigeminal nociception via conditioned pain modulation in persons with migraine headaches. Pain Med (United States) 20(8):1600–1610. https://doi.org/10.1093/pm/pny305
Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? www.elsevier.com/locate/pneurobio
Di Antonio S, Arendt-Nielsen L, Ponzano M, et al. Profiling migraine patients according to clinical and psychophysical characteristics: a cluster analysis approach. Pain Medicine. Published online May 16, 2023. https://doi.org/10.1093/pm/pnad048
Liang Z, Thomas L, Jull G, Minto J, Zareie H, Treleaven J (2021) Neck pain associated with migraine does not necessarily reflect cervical musculoskeletal dysfunction. Headache 61(6):882–894. https://doi.org/10.1111/head.14136
Hvedstrup J, Kolding LT, Younis S, Ashina M, Schytz HW (2020) Ictal neck pain investigated in the interictal state – a search for the origin of pain. Cephalalgia 40(6):614–624. https://doi.org/10.1177/0333102419896369
Hvedstrup J, Kolding LT, Ashina M, Schytz HW (2020) Increased neck muscle stiffness in migraine patients with ictal neck pain: a shear wave elastography study. Cephalalgia 40(6):565–574. https://doi.org/10.1177/0333102420919998
Carvalho GF, Luedtke K, Szikszay TM, Bevilaqua-Grossi D, May A (2021) Muscle endurance training of the neck triggers migraine attacks. Cephalalgia 41(3):383–391. https://doi.org/10.1177/0333102420970184
Burstein R, Cutrer MF, Yarnitsky D (2000) The development of cutaneous allodynia during a migraine attack. Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123(8):1703–1709. https://doi.org/10.1093/brain/123.8.1703
Funding
Open access funding provided by Aalborg University Library
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121). Dr. Pelosin has received research support from JPND Neurodegenerative Disease Research EU joint program and from The Michael J. Fox Foundation. Dr. Bovis reports personal fees from Eisai, personal fees from Novartis, during the conduct of the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Antonio, S., Arendt-Nielsen, L., Ponzano, M. et al. Profiling migraine patients according to clinical and psychophysical characteristics: clinical validity of distinct migraine clusters. Neurol Sci 45, 1185–1200 (2024). https://doi.org/10.1007/s10072-023-07118-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07118-8