Abstract
Introduction
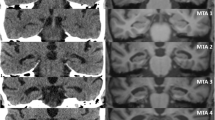
Research using magnetic resonance imaging (MRI) suggests regional cerebral atrophy measures (e.g., frontal lobe, temporal lobe) may predict post-stroke outcomes. Clinical CT scans have excellent potential for use in research but it is unclear whether regional atrophy measures from CT are reliable compared to MRI reference standards.
Methods
We used the Global Cortical Atrophy (GCA) scale to investigate reliability of atrophy measures on CT versus MRI scans from stroke patients originally recruited to the Oxford Cognitive Screening programme. Two raters provided standardised visual ratings at two timepoints. Weighted Kappa statistics assessed the reliability of regional atrophy scores. Spearman’s correlation and a two-way repeated measures ANOVA assessed the reliability of the total score.
Results
On clinically acquired neuroimaging from 98 stroke patients (mean/SD age = 70.97/11.99, 42 female, 84 ischaemic stroke), regional GCA scores on CT versus MRI showed fair to almost perfect intra-rater agreement (κ = .50–.87), substantial to almost perfect intra-rater agreement on CT (κ = .67–.88), and moderate to almost perfect intra-rater reliability on MRI (κ = .50–.89). Regional GCA scores showed mostly moderate to substantial inter-rater reliability on both CT and MRI (κ = .43–.69), except the temporal horns and parieto-occipital region. There was a strong correlation between total GCA scores on CT and MRI (r (96) = .87–.88, p < .001).
Conclusions
These results support the use of cerebral atrophy measures from CT in clinical research, as visual ratings showed generally good agreement between CT and MRI, between raters, and between timepoints.
Similar content being viewed by others
Introduction
Magnetic resonance imaging (MRI) is the favoured imaging modality in research studies due to its higher spatial resolution and improved soft tissue contrast, compared to computed tomography (CT; [21]. However, CT is the standard imaging method in many clinical settings, as it offers substantial time and cost advantages, and it is better tolerated by acutely unwell patients, such as patients with stroke [21].
The large numbers of routinely performed clinical CT scans have great potential for use in research studies that seek to understand the relationship between neuroimaging factors and clinical outcomes in patient populations [8]. Previous studies in stroke patients have employed routinely acquired CT for lesion-symptom mapping [13] and clinical risk prediction [16, 20], and increasing research is focused on the relationship between CT-derived measures of cerebral atrophy and post-stroke outcomes [1].
The relationship between CT-derived cerebral atrophy measures and post-stroke outcomes has mostly been assessed using global cerebral atrophy measures [1]. However, evidence from other imaging modalities—including MRI, diffusion-weighted imaging (DWI), and positron emission tomography (PET)—indicates that regional cerebral atrophy may better predict important post-stroke outcomes, such as performance on the Mini Mental State Examination [11] and development of post-stroke dementia [22]. Determining whether these findings can be replicated using routinely performed clinical CT scans is of key clinical and academic interest, as capitalising on the large numbers of routinely acquired CT scans would allow for more sensitive studies in larger and more representative patient samples.
So far, to our knowledge, it has not been established whether regional cerebral atrophy measures can be reliably derived from CT and whether these are comparable to MRI reference standards. Previous studies have reported moderate to excellent agreement between visual ratings of global atrophy, deep atrophy, superficial atrophy, and medial temporal lobe atrophy on CT versus MRI [8, 18, 19]. However, it remains uncertain whether reliable estimates of cerebral atrophy in key brain regions—such as the frontal lobe, parieto-occipital lobe, and temporal horns—can be derived from routinely acquired CT imaging.
The present study therefore sought to determine using clinically acquired paired CT and MRI scans from stroke patients: (i) the agreement between CT versus MRI ratings of regional cerebral atrophy, and (ii) intra- and inter-rater agreement for regional cerebral atrophy measures on CT and MRI. We also sought to confirm the correlation between total cerebral atrophy measures on CT versus MRI shown by previous research (e.g., [8].
Methods
Participants
This study analysed data collected within the Oxford Cognitive Screening (OCS) programme [6, 7], which recruited a consecutive sample of stroke survivors during acute hospitalisation, assessed domain-specific cognition, and collected routinely acquired clinical brain imaging. Participants were recruited for the OCS programme from Oxford University Hospital’s acute stroke unit between 2016 and 2020. The OCS programme included all patients with a confirmed diagnosis of stroke, who were at least 18 years of age, were able to remain alert for 20 min, and were able to provide informed consent.
The present investigation included all patients recruited for the OCS programme who had undergone both routine clinical CT and MRI brain imaging during the acute/sub-acute stage of stroke (i.e., within 4 weeks post-stroke). A total of 98 patients met these inclusion criteria. Table 1 presents demographic and clinical details of the resultant sample of 98 stroke patients, as recorded by relevant medical records.
Brain imaging
CT scans were non-enhanced (slice thickness 5 mm) and MRI scans were either T1- or T2-weighted, depending on clinical availability. If a patient had both T1- and T2-MRI available, we analysed the highest quality scan (i.e., scan with least imaging artefacts and noise).
Visual rating of regional cerebral atrophy
Two experienced neuroimaging researchers (GH and EC) visually rated cerebral atrophy on axial CT and MRI head scans independently, while blinded to demographic and diagnostic information. Both raters were also blind to MRI visual ratings at the time of CT analysis and vice versa. Both raters performed visual ratings twice on the complete imaging dataset at an interval of at least 2 weeks. Both raters were blind to previous ratings during the second rating session.
For visual ratings, both raters used the Global Cortical Atrophy (GCA) scale, first devised by Pasquier et al. [14]. This scale evaluates cerebral atrophy in 13 brain regions, with most regions evaluated separately in each hemisphere (Table 2). The scale rates atrophy in each region on a 0–3 scale, with scores corresponding to absent (0), mild (1), moderate (2), and severe (3) atrophy [14]. If a region was severely affected by a stroke lesion so that cerebral atrophy could not be evaluated with confidence, the affected region scored the same as the anatomically homologous region in the opposite hemisphere. We also calculated a measure of total cerebral atrophy by summing the scores from all regions (range = 0–39) [14].
Statistical analysis
We used weighted Kappa values with linear weighting to determine the intra-rater, inter-rater, and inter-modality (CT versus MRI) reliability of regional atrophy ratings. Agreement was interpreted according to Landis and Koch: < 0 = less than chance agreement; 0.01–0.20 = slight agreement; 0.21–40 = fair agreement; 0.41–0.60 = moderate agreement; 0.61–0.80 = substantial agreement; 0.81–0.99 almost perfect agreement [10].
In addition, we investigated the agreement between total GCA scores on CT versus MRI by performing a Spearman correlation. We also used a 2 raters × 2 imaging modalities repeated measures ANOVA to investigate differences between CT and MRI ratings for the paired scans by the two raters. As both raters conducted CT and MRI ratings at two timepoints, we analysed CT-MRI agreement using ratings from the first timepoint only.
Data availability
All visual ratings used in the analyses of the present study are openly available on the Open Science Framework (https://osf.io/y8q7n/). CT and MRI scans cannot be shared openly, due to research governance restrictions on the use of clinical imaging data.
Results
CT scans were performed on average 0.49 days after hospitalisation for stroke (SD = 1.33, range = 0–7). MRI scans were performed on average 2.61 days after stroke (SD = 3.44, range = 0–26). CT and MRI scans were separated by on average 2.31 days (SD = 3.33, range = 0–26). Of the 98 paired CT and MRI scans, CT was obtained before, after, and on the same day as MRI in 75, 4, and 19 cases, respectively. Of the 98 MRI scans, 30 scans were T1-weighted and 68 scans were T2-weighted.
CT versus MRI
Weighted Kappas indicated moderate to substantial agreement between regional measures of sulcal atrophy on CT and MRI for both raters (κ = 0.50–0.61). CT-MRI agreement was substantial to almost perfect for regional measures of ventricular atrophy (κ = 0.64–0.87). When intra-rater reliability was averaged across raters, the highest CT-MRI agreement was for the frontal horns and occipital horns, and the lowest CT-MRI agreement was for the parieto-occipital region. Finally, the total cerebral atrophy measure showed fair agreement between CT and MRI for both raters (κ = 0.24–0.35). CT-MRI ratings are plotted by region in Fig. 1. Figure 2 provides all CT-MRI weighted Kappa values by region.
Weighted Kappa statistics for regional cerebral atrophy measures. Weighted Kappa statistics are shown for intra-rater reliability on CT versus MRI, intra- and inter-rater reliability on CT, and intra- and inter-rater reliability on MRI. Colour coding corresponds to Landis and Koch interpretations of weighted Kappa (see “Methods”)
We further investigated CT-MRI agreement for the total GCA score using Spearman’s correlation and a two-way repeated measures ANOVA with scan modality (CT versus MRI) and rater (GH versus EC) as within-subject variables and total GCA score as the dependent variable. There was a strong correlation between total GCA scores on CT and MRI (r (96) = 0.87, p < 0.001 for both raters). The two-way repeated measure ANOVA showed there was a significant difference in total GCA score depending on scan modality (F (1, 97) = 6.52, p < 0.001), but the effect size was small (η2G = 0.003). The total GCA score was significantly lower (i.e., atrophy was quantified as less severe) for CT scans (M = 14.13, SD = 10.39) than for MRI scans (M = 14.94, SD = 9.63). There was also a significant difference in total GCA score depending on rater (F (1, 97) = 46.96, p < 0.001), but the effect size was also small (η2G = 0.017). Total GCA scores from GH (M = 13.58, SD = 10.28) were lower than total GCA scores from EC (M = 15.49, SD = 9.63) (Fig. 3).
CT versus CT (intra-rater)
Intra-rater agreements for regional measures of sulcal atrophy on CT were in the range κ = 0.67–0.82 for both raters, indicating substantial to almost perfect agreement for all sulcal regions. Intra-rater agreement for regional measures of ventricular atrophy on CT was also substantial to almost perfect for both raters (κ = 0.72–0.88). When intra-rater reliability was averaged across raters, the highest intra-rater agreement was for the occipital horns, and the lowest intra-rater agreement was for the temporal horns. Intra-rater agreement for the global cerebral atrophy measure was substantial for both raters (κ = 0.62–0.69) (Fig. 2).
CT versus CT (inter-rater)
Inter-rater agreements for regional measures of sulcal atrophy on CT were in the range κ = 0.43–0.69, indicating moderate to substantial inter-rater agreement for all sulcal regions. Inter-rater agreement for regional measures of ventricular atrophy on CT was fair to substantial for all regions (κ = 0.24–0.79). The highest inter-rater agreement was for the third ventricle (κ = 0.79), and the lowest inter-rater agreement was for the temporal horns (κ = 0.24). Inter-rater agreement for the total cerebral atrophy measure on CT was fair (κ = 0.35) (Fig. 2).
MRI versus MRI (intra-rater)
Intra-rater agreements for regional measures of sulcal atrophy on MRI were in the range κ = 0.50–0.81 for both raters, indicating moderate to almost perfect agreement for all sulcal regions. Intra-rater agreement for regional measures of ventricular atrophy on MRI was substantial to almost perfect for both raters (κ = 0.75–0.89). When intra-rater reliability was averaged across raters, the highest intra-rater agreement was for the third ventricle and the lowest intra-rater agreement was for the parieto-occipital region. Intra-rater agreement for the total cerebral atrophy measure was moderate to substantial for both raters (κ = 0.47–0.65) (Fig. 2).
MRI versus MRI (inter-rater)
Inter-rater agreements for regional measures of sulcal atrophy on MRI were in the range κ = 0.34–0.64, indicating fair to substantial inter-rater agreement for all sulcal regions. Inter-rater agreement for regional measures of ventricular atrophy on MRI was fair to almost perfect for all regions (κ = 0.36–0.82). The highest inter-rater agreement was for the third ventricle (κ = 0.82) and the lowest inter-rater agreement was for the parieto-occipital region (κ = 0.34). Inter-rater agreement for the total cerebral atrophy measure on MRI was fair (κ = 0.24) (Fig. 2).
Discussion
Using paired clinically acquired CT and MRI scans from the acute/subacute stage post-stroke, the present study found that standardised visual ratings of regional cerebral atrophy showed generally good agreement between CT and MRI, between raters, and between timepoints. This is the largest study to date to our knowledge examining CT-MRI agreement for visual ratings of cerebral atrophy and the first study to demonstrate the intra- and inter-rater reliability of fine-grained regional atrophy measures on CT. By demonstrating that atrophy measures from CT are comparable to those from MRI, and that intra- and inter-rater reliability on CT- versus MRI-derived visual ratings are similar, our findings support the use of routinely acquired CT imaging for clinical research studies investigating the relationship between cerebral atrophy and clinical outcomes in patient populations.
First, regional measures of cerebral atrophy showed moderate to almost perfect agreement on CT and MRI. This extends the results of previous studies that investigated CT-MRI agreement for measures of global cerebral atrophy using less fine-grained visual rating scales. Wattjes et al. [19] reported excellent intra-rater agreement between measures of global cerebral atrophy on CT and MRI in a sample of memory clinic patients (n = 30, mean weighted Kappa = 0.83). Similarly, Ferguson et al. [8] reported moderate-to-substantial intra-rater agreement between CT and MRI for measures of superficial atrophy and deep atrophy in a sample of stroke patients (n = 70). While global cerebral atrophy has been shown to predict important post-stroke clinical outcomes, such as futile recanalisation [12], future studies may benefit from a more detailed region-specific approach to atrophy assessment, particularly studies that seek to better understand risk factors for cognitive impairment after stroke [11, 15, 22]. The present study provides a fundamental basis for future research by demonstrating that regional atrophy measures on CT are broadly comparable to those derived from higher resolution MRI, and thus suitable for use in clinical research.
Although CT-MRI agreement was moderate to almost perfect for regional atrophy measures, agreement was lower for total atrophy measures. Nevertheless, we observed a strong correlation between total GCA ratings on CT and MRI, which suggests that the lower CT-MRI agreement for the total cerebral atrophy ratings may have been driven by systematic differences in visual ratings on CT versus MRI. This is supported by the results of the two-way repeated measures ANOVA, which showed a trend towards higher total atrophy scores on MRI versus CT for both raters. Although the cause of this systematic difference in scoring is not entirely clear, it is possible that atrophy appeared superficially worse on MRI because of its higher resolution compared to CT [21]. Alternatively, MRI may be more sensitive to atrophy in certain brain regions - for example, the temporal lobe, which is frequently obscured by hyperintense bone on CT imaging. Higher scores on MRI may stem from such increased regional sensitivities, as the total GCA score in the present study was calculated by summation of regional scores.
The present study demonstrated mostly substantial to almost perfect intra- and inter-rater reliability for regional cerebral atrophy ratings on CT. The only exception to this was inter-rater reliability for temporal horn atrophy on CT. Nevertheless, intra-rater reliability for temporal horn atrophy on CT was still substantial, which suggests that the rating scale was applied differently by the two trained raters for this brain region. Therefore, the relatively low inter-rater reliability for temporal horn atrophy could be mitigated in future studies by providing more specific operational definitions and/or exemplars for each temporal horn atrophy score. However, as stated earlier, temporal horn evaluation may be impacted by the presence of hyperintense bone effects. Therefore, although the GCA scale was specifically designed for application on axial slices, future studies may obtain more reliable assessments of temporal horn atrophy on coronal CT slices.
We found lower CT-MRI agreement and lower inter-rater agreement on CT for measures of total cerebral atrophy, compared to previous studies that investigated the reliability of total cerebral atrophy measures on CT and MRI [8, 19]. This is likely due to several differences between our study and previous studies. Firstly, our measurement of total cerebral atrophy included a larger range of available scores (range = 0–39) than previous studies (e.g., range = 0–3; [19]. As the magnitude of Kappa is influenced by the number of categories in the measurement scale, this is likely to have affected our Kappa values [17]. Secondly, our measure of total atrophy may have been impacted by the presence of acute stroke lesions. Because we allocated any region obscured by stroke the same score as the homologous region in the opposite hemisphere, any small discrepancies in regional scoring may have inflated differences in the total measure. Despite these differences, however, our analysis still demonstrated fair CT-MRI agreement and inter-rater agreement for total atrophy measures.
Our findings support the use of regional cerebral atrophy measures from CT for future research in patient populations. CT is the most common imaging modality in acute hospital admissions for stroke, so there is great untapped potential for these scans to be used in research studies investigating the relationship between CT-derived neuroimaging measures and post-stroke outcomes. A particularly interesting avenue for future research is the relationship between regional cerebral atrophy and domain-specific cognitive functioning after stroke, given the clearly demonstrated association between both temporal lobe atrophy and memory, and between frontal lobe atrophy and executive impairments [4]. Furthermore, as localised forms of cerebral atrophy radiologically characterise various dementias [2, 3, 5, 9], future studies should investigate the association between CT-derived regional cerebral atrophy measures and post-stroke dementia. If CT-derived regional atrophy measures are reliably associated with post-stroke cognitive outcomes, cerebral atrophy measures could be incorporated into clinical risk prediction algorithms to help identify patients at risk of post-stroke cognitive impairment and/or post-stroke dementia. This would be particularly valuable should automated atrophy assessment tools become widely available for CT imaging.
There were some limitations in the present study. Firstly, as we used routine clinically acquired CT and MRI brain scans, these often included mild motion artefacts and suboptimal axial slice angulation. This may explain some of the discrepancy in regional cerebral atrophy measures on CT and MRI. Nevertheless, demonstrating the reliability of the GCA scale in a clinically representative sample with ‘imperfect’ brain scans adds substantially to the clinical relevance of the present study. Secondly, we analysed only T1- and T2-MRI so it is not clear whether CT-derived measures of regional cerebral atrophy agree equally well with measures from other MRI sequences, such as fluid attenuated inversion recovery sequences.
Overall, the present study demonstrated that regional cerebral atrophy measures show good agreement between CT and MRI. Furthermore, intra- and inter-rater agreements for regional atrophy measures from CT were broadly compared to measures from MRI. The present study therefore supports the use of routinely acquired CT brain imaging in research, as utilising clinically acquired CT scans will enable important clinical questions to be investigated in highly representative patient samples.
References
Ball EL, Sutherland R, Squires C, Mead GE, Religa D, Lundström E, Cheyne J, Wardlaw JM, Quinn TJ, Shenkin SD (2021) Predicting post-stroke cognitive impairment using acute CT neuroimaging: a systematic review and meta-analysis. Int J Stroke, 17474930211045836. https://doi.org/10.1177/17474930211045836
Boccardi M (2003) The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging 24(1):95–103. https://doi.org/10.1016/S0197-4580(02)00045-3
Boccardi M, Laakso MP, Bresciani L, Galluzzi S, Geroldi C, Beltramello A, Soininen H, Frisoni GB (2003) The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging 24(1):95–103. https://doi.org/10.1016/S0197-4580(02)00045-3
Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW (2011) Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging 32(4):572–580. https://doi.org/10.1016/j.neurobiolaging.2009.04.011
Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN (2001) Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology 57(10):1756–1763. https://doi.org/10.1212/WNL.57.10.1756
Demeyere N, Riddoch MJ, Slavkova ED, Bickerton W-L, Humphreys GW (2015) The Oxford Cognitive Screen (OCS): Validation of a stroke-specific short cognitive screening tool. Psychol Assess 27(3):883–894. https://doi.org/10.1037/pas0000082
Demeyere N, Riddoch MJ, Slavkova ED, Jones K, Reckless I, Mathieson P, Humphreys GW (2016) Domain-specific versus generalized cognitive screening in acute stroke. J Neurol 263(2):306–315. https://doi.org/10.1007/s00415-015-7964-4
Ferguson KJ, Cvoro V, MacLullich AMJ, Shenkin SD, Sandercock PAG, Sakka E, Wardlaw JM (2018) Visual rating scales of white matter hyperintensities and atrophy: comparison of computed tomography and magnetic resonance imaging. J Stroke Cerebrovasc Dis 27(7):1815–1821. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.02.028
Kirshner HS, Lavin PJM (2006) Posterior cortical atrophy: a brief review. Curr Neurol Neurosci Rep 6(6):477–480. https://doi.org/10.1007/s11910-006-0049-0
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174. https://doi.org/10.2307/2529310
Mok V, Chang C, Wong A, Lam WWM, Richards PS, Wong KT, Wong KS (2005) Neuroimaging determinants of cognitive performances in stroke associated with small vessel disease. J Neuroimaging 15(2):129–137. https://doi.org/10.1111/j.1552-6569.2005.tb00297.x
Monteiro A, Waqas M, Rai HH, Baig AA, Dossani RH, Cappuzzo JM, Levy EI, Siddiqui AH (2022) The impact of brain atrophy on the outcomes of mechanical thrombectomy. Br J Radiol 95(1132):20210494. https://doi.org/10.1259/bjr.20210494
Moore MJ, Demeyere N (2022) Lesion symptom mapping of domain-specific cognitive impairments using routine imaging in stroke. Neuropsychologia 167:108159. https://doi.org/10.1016/j.neuropsychologia.2022.108159
Pasquier F, Leys D, Weerts J, Mounier-Vehier F, Barkhof F, Scheltens P (1996) Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 36:268–272. https://doi.org/10.1159/000117270
Pendlebury ST, Rothwell PM (2009) Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 8(11):1006–1018. https://doi.org/10.1016/S1474-4422(09)70236-4
Pendlebury ST, Thomson RJ, Welch SJV, Kuker W, Rothwell PM, for the Oxford Vascular Study (2022) Utility of white matter disease and atrophy on routinely acquired brain imaging for prediction of long-term delirium risk: population-based cohort study. Age Ageing 51(1):200. https://doi.org/10.1093/ageing/afab200
Sim J, Wright CC (2005) The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 85(3):257–268
Simoni M, Li L, Paul NLM, Gruter BE, Schulz UG, Küker W, Rothwell PM (2012) Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients. Neurology 79(12):1215–1222. https://doi.org/10.1212/WNL.0b013e31826b951e
Wattjes MP, Henneman WJP, van der Flier WM, de Vries O, Träber F, Geurts JJG, Scheltens P, Vrenken H, Barkhof F (2009) Diagnostic imaging of patients in a memory clinic: comparison of MR imaging and 64–detector row CT. Radiology 253(1):174–183. https://doi.org/10.1148/radiol.2531082262
Weaver NA, Kuijf HJ, Aben HP, Abrigo J, Bae H-J, Barbay M, Best JG, Bordet R, Chappell FM, Chen CPLH, Dondaine T, van der Giessen RS, Godefroy O, Gyanwali B, Hamilton OKL, Hilal S, Huenges Wajer IMC, Kang Y, Kappelle LJ, … Biessels GJ (2021) Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 20(6):448–459. https://doi.org/10.1016/S1474-4422(21)00060-0
Wippold FJ (2007) Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging 25(3):453–465. https://doi.org/10.1002/jmri.20838
Yang J, Wong A, Wang Z, Liu W, Au L, Xiong Y, Chu WWC, Leung EYL, Chen S, Lau C, Chan AYY, Lau AYL, Fan F, Ip V, Soo Y, Leung T, Ho CL, Wong LKS, Mok VCT (2015) Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimer’s Dementia 11(1):16–23. https://doi.org/10.1016/j.jalz.2014.01.003
Acknowledgements
This work was supported by OUP John Fell Funding HMD00460 and NIHR RCF grant number NIHR RCF 21/040) and awards from the Stroke Association to ND (SA PPA 18/100032) and ESRC doctoral training funding to GH. STP is supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC) based at Oxford University Hospitals NHS Trust. The OCS study was supported by the National Institute for Health Research Clinical Research Network. We would like to thank Dr. Margaret Moore and Ms. Romina Basting for contributions to curating the scan data employed in this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study retrospectively analysed data from the OCS programme, which received ethical approval from the NHS (OCS-Table and OCS-Recovery studies: NHS RECs 14/LO/0648 and 18/SC/0550).
Informed consent
All participants in the OCS programme provided informed or witnessed consent to participate in the study. This included consent for any brain scans acquired as part of routine care to be analysed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hobden, G., Colbourne, E., Pendlebury, S.T. et al. Reliability of the global cortical atrophy visual rating scale applied to computed tomography versus magnetic resonance imaging scans in acute stroke. Neurol Sci 45, 1549–1556 (2024). https://doi.org/10.1007/s10072-023-07113-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07113-z