Abstract
The mutations on microtubule associated protein tau (MAPT) gene manifest clinically with behavioural frontotemporal dementia (FTD), parkinsonism, such as progressive supranuclear palsy and corticobasal degeneration, and rarely with amyotrophic lateral sclerosis (ALS). FTD-parkinsonism and FTD-ALS are clinical overlaps included in the spectrum of MAPT mutation’s phenotypes. The mutations on MAPT gene cause the dysfunction of tau protein determining its accumulation in neurofibrillary tangles. Recent data describe frequently the co-occurrence of the aggregation of tau protein and α-synuclein in patients with parkinsonism and Parkinson disease (PD), suggesting an interaction of the two proteins in determining neurodegenerative process. The sporadic description of PD-ALS clinical complex, known as Brait–Fahn–Schwarz disease, supports the hypothesis of common neuropathological pathways between different disorders. Here we report the case of a 54-year-old Italian woman with idiopathic PD later complicated by ALS carrying a novel MAPT variant (Pro494Leu). The variant is characterized by an amino acid substitution and is classified as damaging for MAPT functions. The case supports the hypothesis of tau dysfunction as the basis of multiple neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The overlap of clinical syndromes is not rare, especially among neurodegenerative disorders that share neuropathological features and genetic determinants. Frontotemporal dementia (FTD)-parkinsonism and amyotrophic lateral sclerosis (ALS)-FTD are typical examples [1, 2]. Instead, the association between Parkinson disease (PD) and ALS, a complex termed Brait–Fahn–Schwarz disease (BFS) is extremely rare [3,4,5,6,7,8]. After the first description [3], few cases have been sporadically reported worldwide [4,5,6,7,8]. Neuropathological information on BFS patients is scarce and controversial [3,4,5,6,7,8], and a genetic link has not been identified so far.
Here, we report the first case of a 54-year-old Italian woman affected by BFS disease (PD-ALS) carrying a novel variant of the microtubule associated protein tau (MAPT) gene.
Materials and methods
Genetics: Genomic DNA was isolated from peripheral blood sample using a standard automatic method (QIAcube, QIAGEN). Initially, MAPT gene and progranulin (GRN) gene were PCR amplified and analysed by Sanger sequencing on SeqStudio Genetic Analyzer (Thermo Fisher, Waltham, MA, USA). Subsequently, a more thorough sequencing of the patient DNA was performed with a custom next-generation sequencing (NGS) panel of 73 dementia-related genes. Sample library was prepared using the Illumina® DNA Prep with Enrichment kit. Reads were aligned to GRCh37/hg19 reference genome. Variants were annotated with ANNOVAR [9], and allele frequency was checked on population databases (1000Genomes, ESP, and gnomAD) [10]. In silico prediction tools (SIFT, PolyPhen-2, REVEL, Mutation Assessor, CADD, and MetaLR) were used to assess non-synonymous variants and their possible effect on protein structure and function. Moreover, the apolipoprotein E (APOE) haplotype was investigated by high-resolution melting analysis (HRMA). The regions encompassing rs7412 [NC_000019.9:g.45412079C>T] and rs429358 (NC_000019.9:g.45411941T>C) of APOE were PCR amplified using two sets of designed primers. Samples with known APOE haplotype were used as standard references.
Case report
Clinical features
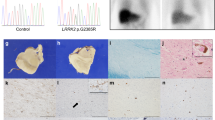
A 54-year-old healthy woman developed resting tremor of the left hand, then tremor of the left leg, and slight bradykinesia of the left limbs. Brain magnetic resonance imaging (MRI) detected mild microvascular ischemic changes of the white matter, while the dopamine transporter brain imaging with single-photon emission computed tomography (DAT-SPECT) revealed reduced uptake in both putamen nuclei, mostly in right putamen (Fig. 1). The 123I-metaiodobenzylguanidine (MIBG) myocardial scintigraphy showed an impairment of the cardiac sympathetic nerve fibres. Electromyography (EMG) examination and motor evoked potentials (MEP) were normal, as well as the cognitive profile. The patient was treated with rasagiline 1 mg a day and long-lasting pramipexole up to 2.1 mg a day. The treatment was effective and well tolerated. Based on PD diagnostic criteria [11], a diagnosis of idiopathic PD was made. After 2 years of clinical stability, the patient complained frequent falls due to weakness of lower limbs. Gait became rapidly difficult, and at the age of 58 years, she could stand up only with help and could perform only few steps. Six months later, she was bedridden due to progressive weakness of the four limbs. Tendon reflexes were increased in upper and lower limbs, and Hoffmann and Babinski signs were bilaterally present. Increased muscle tone, with both spasticity and rigidity, was detected in the four limbs, with prevalence on the left side. Muscular atrophy and fasciculations were observed on both deltoids and quadriceps muscles. Her speech was dysarthric and hypophonic. Brain MRI resulted unchanged and cervical MRI ruled out spinal lesions. Total body CT scan with contrast medium did not detect any abnormalities in the brain, neck, chest, and abdomen. Liver, kidney, renal, and thyroid functions were normal. An infective assessment was performed in the blood (HCV, HBV, syphilis, Borrelia, HIV, HTLV) and in the cerebrospinal fluid (syphilis, HSV, EBV, VZV, Borrelia). No infections were detected. The screening for autoantibody included anti-Hu, anti-Yo, anti-Ri, anti-CV2, anti-Anfifisina, anti-NMDA, anti-LG1, anti-CASPR, anti-GABA, anti-GluR1, anti-GluR2, and anti-gangliosides (GQ1b, GT1b, GT1a, GD3, GD1b, GD1a, GM3, GM2, GM1) and gave negative results. Electrodiagnostic studies showed reduction of compound muscle action potential (CMAP) amplitudes in most motor nerves examined (right and left tibial, peroneus, median, and ulnar nerves) from 4 limbs, with normal conduction velocity. Sensory nerve conduction study was normal. Standard needle EMG examination, performed in the bulbar and thoracic regions, and at proximal and distal muscles from each limb, showed diffuse fibrillation and fasciculation potentials in the upper and lower extremities, associated to chronic motor unit changes. MEPs, measured from posterior tibial and ulnar nerves, resulted of reduced amplitude as compared to distal CMAP values, with increased central conduction time. A diagnosis of definite ALS was made according to revised El Escorial criteria [12]. Cognitive evaluation was not performed due to the severe dysarthria; however, the patient was oriented in time, space and toward people, but apathy, loss of empathy, and inappropriate laughing and crying were detected. Cerebrospinal fluid was normal including neurodegenerative biomarkers of amyloid and tau protein: Aβ42 660 pg/ml (n.v >670 pg/ml); Aβ42/Aβ40 0.10 (n.v > 0.062) ; total-tau 247 pg/ml (n.v < 400 pg/ml); phosphorylated-tau 181 13.3 pg/ml (n.v.< 60 pg/ml) [13]. A conclusive diagnosis of ALS associated with PD was made. To complete the diagnostic process, genetic analysis with a panel of 73 dementia-related genes was performed, including genes associated with FTD and ALS (GRN, C9orf72, SOD1, TARDBP, MAPT). After the discharge from the hospital, the clinical condition of the patient rapidly deteriorated, and swallowing and breathing difficulties required the insertion of a gastric tube and the use of non-invasive ventilation. Ten months later, at the age of 59 years, she died of respiratory failure. A request for autopsy was declined by family members.
The dopamine transporter brain imaging with single-photon emission computed tomography (DAT-SPECT) revealing reduced uptake in both putamen nuclei, mostly in right putamen. Volume distribution (VD): right caudate 2.33, left caudate 2.35, right putamen 0.71, left putamen 0.88 (normal VD values: right caudate 2.58±0.41; left caudate 2.57±0.37; right putamen 2.14±0.41; left putamen 2.23±0.45)
Genetic features
The patient was found to carry a novel missense variant in exon 9 of MAPT gene, NM_016835.4:c.1481C>T, p.(Pro494Leu). The p.(Pro494Leu), rs763728305 indbSNP, is classified as variant with an uncertain significance according to the American College of Medical Genetics and Genomics guidelines. SIFT and PolyPhen-2 predict that the variant is deleterious/possible damaging for protein structure and function (SIFT score= 0.01, scores <0.05 are predicted to influence protein activity; PolyPhen-2 score= 0.465, score ranges from 0.0=tolerated to 1.0=deleterious), instead REVEL, Mutation Assessor, and MetaLR evaluate the variant as likely benign/tolerated. The residue is conserved (phyloP100way = 6.033, greater than 3.81 cutoff). CADD v1.6 indicate a 22 score; C-score≥ 20 indicates the 1% most deleterious substitution in human genome. The variant p.(Pro494Leu) was not reported in ClinVar database and has a frequency in general population of ƒ = 0.00000398 (GnomAD). Sequencing not evidenced other significant variants in the patient, APOE haplotype resulted ε3/ε3. Family history was negative for neurological disorders: the father died at 94 years, while the mother died at young age at 54 years of haematological neoplasm. The patient’s relatives, a healthy 5-year-old brother and two daughters, preferred not to undergo the genetic testing.
Discussion
We presented the case of 54-year-old Italian woman with levodopa-responsive parkinsonism who later developed clinical symptoms consistent with ALS. The initial clinical suspicion of idiopathic PD was supported by the responsiveness to levodopa and the impairment of sympathetic innervation at myocardial scintigraphy, a method used to differentiate between atypical parkinsonism and PD [14]. Moreover, the phenotype of MND fitted the revised El Escorial criteria for ALS [12]. The clinical picture resembles the phenotype of the BFS disease [3] characterized by PD followed after 1–2 years by ALS. The BFS disease has been described in few case reports [3, 5, 6, 8] and screened in two cohort studies [4, 7], for a total of no more than 50 cases since its first description in 1973 [3]. In the first cohort study, 27 of 5.500 patients with parkinsonism also had MND, only seven of whom had PD-ALS complex [4]. In the other cohort study, among 1042 patients diagnosed with ALS or other MNDs, six had concomitant diagnosis of PD [7].
No evidence of mutations in genes associated with FTD-ALS (SOD1, FUS, angiogenin, GRN) nor with PD (Park-1, Park-2, Park-6, Park-7, DJ-1) were detected so far. MAPT gene was not screened in the total of the cases [5, 6, 8]. Pasquini et al. reported one mutation in C9orf72 gene and one in TARDP gene, but it is not specified if they refer to patients with atypical parkinsonism-MND or with PD-ALS [7]. Our patient was found to carry a novel variant of MAPT gene causing an amino acid substitution in exon 9 with a potential pathological role, as predicted by the in silico prediction tools.
The MAPT gene encodes for protein Tau and exon 9 is placed in the microtubule (MT)-binding domain. Mutations in the MT-binding domain resulted in microtubules destabilization, unbound tau, and consequently tau aggregation [15]. Neurodegenerative disorders associated with MAPT mutations are bvFTD, primary progressive aphasia, progressive supranuclear palsy, corticobasal degeneration, and the FTD-parkinsonism and FTD-ALS clinical complexes [1, 2]. All are characterized by the accumulation of hyperphosphorylated tau (neurofibrillary tangles, NFTs). The role of MAPT in pure ALS is less characterized than in the FTD-ALS complex; however, recently, a MAPT mutation was identified in two unrelated ALS Italian patients [16, 17]. MAPT mutations have never been described in patients with PD, but PD patients have been found to present both α-synuclein and NFTs aggregations [2]. Interestingly, Tau protein, behind its functions related to microtubules, is involved in common hallmarks of neurodegeneration processes, such as increased oxidative stress and mitochondrial dysfunction, suggesting a crucial role of tau in the pathogenesis of different neurodegenerative disorders. In vivo and in vitro data reported that the dysfunction of tau could produce different kinds of proteinopathies [2, 15, 18] The lack of neuropathological data does not allow us to confirm this hypothesis. Moreover, the pathogenicity of this novel MAPT variant is uncertain, as we don’t have information on the genetic status of family members and RNA sample of the patient for molecular analysis was not available.
However, if this variant is not the cause of this clinical complex, we think it can still represent a risk factor. Previous studies have already reported that MAPT haplotype and MAPT variants influence the risk of both PD [2, 18, 19] and ALS [18, 20,21,22]. Even if a MAPT variant is silent at protein level, it can affect the level of tau and the post-transcriptional modifications and can influence the interaction between tau and other proteins [18]. The level of tau and its phosphorylation are supposed to influence the interaction between tau and α-synuclein and their synergistic fibrillization [2, 18, 23].
This is the first case in which a variant of the MAPT gene (Pro494Leu) was associated with a BFS’s phenotype, idiopathic PD followed by ALS. This variant of MAPT gene has never been identified before, and if not, the cause of the syndrome could be, at least, a risk factor, supporting the crucial role of tau in the development of different neurodegenerative disorders.
Data Availability
The data present in this article are available on reasoned request from the corresponding author.
References
Zecca C, Tortelli R, Carrera P, Dell'Abate MT, Logroscino G, Ferrari M (2023) Genotype-phenotype correlation in the spectrum of frontotemporal dementia-parkinsonian syndromes and advanced diagnostic approaches. Crit Rev Clin Lab Sci1 60(3):171–188. https://doi.org/10.1080/10408363.2022.2150833
Leveille E, Ross OA, Gan-Or Z (2021) Tau and MAPT genetics in tauopathies and synucleinopathies. Parkinsonism Relat Disord 90:142–154. https://doi.org/10.1016/j.parkreldis.2021.09.008
Brait K, Fahn S, Schwarz GA (1973) Sporadic and familial parkinsonism and motor neuron disease. Neurology 23(9):990–1002. https://doi.org/10.1212/wnl.23.9.990
Gilbert RM, Fahn S, Mitsumoto H, Rowland LP (2010) Parkinsonism and motor neuron diseases: twenty-seven patients with diverse overlap syndromes. Mov Disord 25(12):1868–1875. https://doi.org/10.1002/mds.23200
Manno C, Lipari A, Bono V, Taiello AC, La Bella V (2013) Sporadic Parkinson disease and amyotrophic lateral sclerosis complex (Brait-Fahn-Schwarz disease). J Neurol Sci 326(1-2):104–106. https://doi.org/10.1016/j.jns.2013.01.009
Belin J, Gordon PH, Guennoc AM, De Toffol B, Corcia P (2015) Brait-Fahn-Schwarz disease: the missing link between ALS and Parkinson’s disease. Amyotroph Lateral Scler Frontotemporal Degener 16(1-2):135–136. https://doi.org/10.3109/21678421.2014.94888016(1-2):135-136
Pasquini J, Trogu F, Morelli C, Poletti B, Girotti F, Peverelli S, Brusati A, Ratti A, Ciammola A, Silani V, Ticozzi N (2022) Parkinsonian syndromes in motor neuron disease: a clinical study. Front Aging Neurosci 14:917706. https://doi.org/10.3389/fnagi.2022.917706
Erol AM, Kilic AK, Celik A, Celik C, Basak AN (2016) Brait-Fahn-Schwarz disease: Parkinson’s disease and amyotrophic lateral sclerosis complex. Acta Neurol Belg 116(3):401–403. https://doi.org/10.1007/s13760-015-0531-z
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38(16):e164. https://doi.org/10.1093/nar/gkq603
Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46:310e315. https://doi.org/10.1038/ng.2892
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. https://doi.org/10.1002/mds.26424
Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, Robberecht W, Shefner J, WFN Research Group On ALS/MND (2015) A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 16(5-6):291–292. https://doi.org/10.3109/21678421.2015.1049183
Alcolea D, Pegueroles J, Muñoz L, Camacho V, López-Mora D, Fernández-León A, Le Bastard N, Huyck E, Nadal A, Olmedo V, Sampedro F, Montal V, Vilaplana E, Clarimón J, Blesa R, Fortea J, Lleó A (2019) Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on Lumipulse. Ann Clin Transl Neurol 6(9):1815–1824. https://doi.org/10.1002/acn3.50873
Orimo S, Suzuki M, Inaba A, Mizusawa H (2012) 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 18:494–500. https://doi.org/10.1016/j.parkreldis.2012.01.009
Strang KH, Golde TE, Giasson BI (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Invest 99(7):912–928. https://doi.org/10.1038/s41374-019-0197-x
Origone P, Geroldi A, Lamp M, Sanguineri F, Caponnetto C, Cabona C, Gotta F, Trevisan L, Bellone E, Manganelli F, Devigili G, Mandich P (2018) Role of MAPT in pure motor neuron disease: report of a recurrent mutation in Italian patients. Neurodegener Dis 18(5-6):310–314. https://doi.org/10.1159/000497820
Di Fonzo A, Ronchi D, Gallia F, Cribiù FM, Trezzi I, Vetro A et al (2014) Lower motor neuron disease with respiratory failure caused by a novel MAPT mutation. Neurology 82(22):1990–1998. https://doi.org/10.1212/WNL.0000000000000476
Zhang CC, Zhu JX, Wan Y, Tan L, Wang HF, Yu JT, Tan L (2017) Meta-analysis of the association between variants in MAPT and neurodegenerative diseases. Oncotarget 8(27):44994–45007. https://doi.org/10.18632/oncotarget.16690
Pastor P, Ezquerra M, Muñoz E, Martí MJ, Blesa R, Tolosa E, Oliva R (2000) Significant association between the tau gene A0/A0 genotype and Parkinson's disease. Ann Neurol 47(2):242–245
Münch C, Prechter F, Xu R, Linke P, Prudlo J, Kuzma M, Kwiecinski H, Ludolph AC, Meyer T (2005) Frequency of a tau genotype in amyotrophic lateral sclerosis. J Neurol Sci 236(1-2):13–16. https://doi.org/10.1016/j.jns.2005.04.004
Fang P, Xu W, Wu C, Zhu M, Li X, Hong D (2013) MAPT as a predisposing gene for sporadic amyotrophic lateral sclerosis in the Chinese Han population. Neural Regen Res 8(33):3116–3123. https://doi.org/10.3969/j.issn.1673-5374.2013.33.005
Petrozziello T, Amaral AC, Dujardin S, Farhan SMK, Chan J, Trombetta BA, Kivisäkk P, Mills AN, Bordt EA, Kim SE, Dooley PM, Commins C, Connors TR, Oakley DH, Ghosal A, Gomez-Isla T, Hyman BT, Arnold SE, Spires-Jones T et al (2022) Novel genetic variants in MAPT and alterations in tau phosphorylation in amyotrophic lateral sclerosis post-mortem motor cortex and cerebrospinal fluid. Brain Pathol 32(2):e13035. https://doi.org/10.1111/bpa.13035
Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM (2003) Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 300(5619):636–640. https://doi.org/10.1126/science.1082324
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.F., A.I. and B.N. Methodology: A.I., S.B., and B.N. Investigation, C.F., A.I., S.M., S.R., L.C., V.B. Resources: C.F., S. M., S.R., L.C. and V.B. Data curation: C.F., A.I., S.M., S.R., S.M., and B.N. Writing—original draft preparation: C.F, A.I. Writing—review and editing: C.F., A.I., S.M., S.R., and B.N. Supervision: B.N. and S.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki.
Informed consent
An oral consent was obtained from the family members to publish the case.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrari, ., Ingannato, A., Matà, S. et al. Parkinson-ALS with a novel MAPT variant. Neurol Sci 45, 1051–1055 (2024). https://doi.org/10.1007/s10072-023-07081-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07081-4