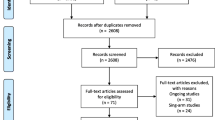
Abstract
Purpose
Stem cells have been extensively used during the last decade to improve clinical outcomes after stroke. The dramatic increase in trials in this field has led us to perform a systematic review and meta-analysis to understand the safety, effectiveness, and relative limitations of this type of intervention.
Method
This review summarizes the current evidence pooled from PubMed (Medline), EMBASE, EBSCOhost, http://clinicaltrials.gov, Scopus (Elsevier), Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science (Science Citation Index Expanded) databases for the use of stem cell therapies in stroke patients without combinations with other treatment modalities. The National Institutes of Health Stroke, modified Rankin Scales, and Barthel Index scores after external stem cell administration have been evaluated on the 3rd, 6th, and 12th months after treatment. The random effect analysis was performed using the Review Manager 5.4.1. The characteristics of stem cell sources and their adverse effects have been discussed as well.
Findings
Although reasonably safe, the effectiveness evidence fluctuated to a large extent due to the heterogeneity of the clinical trials and the absence of a systematic approach. The stem cell sources and the administration window were not strongly associated with clinical outcomes.
Conclusion
Further studies should be conducted to understand the deep discrepancy between preclinical and clinical trials and to execute phase 3 clinical trials with robust control of study characteristics and outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the leading causes of adult death and disability [1]. Despite advances in acute care, there is still no FDA-approved treatment option targeting neuroprotection following acute ischemic stroke, and the only acceptable therapy to promote early reperfusion remains the administration of tissue plasminogen activator and/or mechanical thrombectomy. These, given the short therapeutic window, are used only in the minority of cases and are not a treatment option for hemorrhagic stroke [2]. Even if the treatment protocols are constantly updated given the rapid advancement of endovascular techniques, certain procedures have provided clinical benefit within 6 h after symptoms onset [3]. The brain’s internal capacity to recover after stroke remains limited, although various mechanisms exist. Several trials to enhance the capacity of the brain to combat ischemic stroke were conducted [4]. These include the activation of the brain’s intrinsic defense mechanisms and external administration of stem and progenitor cells. From this perspective, stem cell therapy is an emerging therapeutic approach for stroke treatment. There is now a significant body of evidence coming from the preclinical data and clinical trials that stem cell administration has the potential to modulate multiple pathways arising from stroke. These include microenvironmental changes, reduction of the inflammatory response, and possible replacement of the injured tissue [5]․ However, the fundamental question remains, whether this therapy is safe and to what extent it can contribute to the recovery after stroke. Several systematic reviews were performed to address the effectiveness and usability of stem cell therapies for ischemic stroke. A. M. Abdullahi and colleagues analyzed the data of trials in the case of ischemic stroke, thus excluding hemorrhagic stroke trials, and the analysis included bone marrow-derived stem cell sources [6]. On another systematic approach, the authors broadened the stem cell sources, without explicitly analyzing the data for a specific time period [7]. In our review, we included the stem cell sources regardless of their source and marked the effects at 3-, 6-, and 12-month post-administration both in studies with and without comparator arms. The safety, feasibility, and effectiveness of external stem cell administration were evaluated.
Methods
Search strategy
Two independent reviewers performed the search using preliminarily defined keywords as shown on Table 1. The specific search strategies were created in consultation with a health sciences librarian with expertise in systematic review searches. The keyword selection was based on previously conducted reviews, as well as relevant keyword searches through scientific databases. After the PubMed strategy was finalized, it was adapted to the syntax and subject headings of the other databases. The databases included PubMed (Medline), EMBASE, EBSCOhost, http://clinicaltrials.gov, Scopus (Elsevier), Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science (Science Citation Index Expanded). In the event of differing interpretations, the two investigators re-evaluated the source material independently in an attempt to reach a consensus. If a consensus still could not be reached, a third, independent investigator was brought in to review the material and provide a decisive judgment.
Inclusion/exclusion criteria
The review evaluated the clinical trials, where patients, who had any kind of stroke, received stem cell therapy in the acute or chronic poststroke period, with any administration route, without combination with other therapeutic approaches. The safety measure included adverse effects (Table 2). The effectiveness measures were the National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), and Barthel Index (BI) scores in baseline and at 3rd, 6th, and 12th months after treatment. The inclusion and exclusion criteria are shown in Table 2. The subgroups of the data were studies with and without a comparator arm.
Meta-analysis workflow
The data were analyzed by the Review Manager 5.4.1 [8]. For the studies without a comparator arm, the data were used to calculate the change from baseline at 3, 6, and 12 months after treatment. The mean and the 95% confidence intervals were used to calculate the difference scores. The treatment effects for the controlled studies were explored at 3, 6, and 12 months. The baseline data for each arm was inspected to ensure that the groups did not significantly differ in NIHSS, BI, and mRS scores. To account for the potential heterogeneity across the studies, the random effects model has been used. Pooled estimates were presented as the mean difference (MD) with 95% confidence intervals. The respective forest plots were generated. Heterogeneity was summarized using the I-squared statistic in case of data was available from an adequate number of studies.
Findings
Study selection
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to select the articles [9]. 30 articles, that fulfilled the inclusion criteria, were included for qualitative analysis (Fig. 1) [4, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. 14 studies for NIHSS, 14 studies for BI, and 11 studies for mRS fulfilled the criteria for quantitative synthesis (Fig. 1).
Patient baseline characteristics
All patients, with the exception of those in the study by [39], received acute treatment for stroke based on clinical characteristics and imaging findings. The majority of trials involved patients from both genders with an age range from 3 and 85 years. The mean age of patients (mean ± SD if available), age range, and number or patients in each included study are summarized in supplementary material 1. Among the studies included in our analysis, only the cohort from Tsang KS. et al. (2017) comprised patients with hemorrhagic stroke. Meanwhile, the study from Jiang Y. et al. (2013) had one patient with a hemorrhagic stroke, Chen L. et al. (2013) had four such patients, Suarez-Monteagudo C et al. (2009) had three, and Rabinovich SS et al. (2005) also had three patients. All other patients had ischemic stroke. The most relevant exclusion criteria for the treatment were lacunar syndrome, any severe condition likely to interfere the treatment, malignant disorders, pregnancy, HIV positivity, prior immunosuppression, participation in another investigational drug or device study, contraindication to imaging studies, severe hemorrhagic transformation of the ischemic lesion, and unwillingness to participate (supplementary material 1).
Analysis of the type of stem cells used in studies and study characteristics
The majority of studies (10) have used bone marrow-derived mononuclear cells (BM-MSCs) for the studies [10,11,12,13, 17, 19, 25, 32,33,34, 39], where all of the cell sources were autologous. Eight studies utilized bone marrow-derived mesenchymal stem cells (BM-MesSCs) [13, 15, 16, 18, 26, 28, 29, 38]; six of the cell sources were autologous, while two were allogenic [18, 38]. Remaining trials used CTX-DP immortalized human neural stem cell line [37], multipotent adult stem cells [35], umbilical cord blood MesSCs [22, 23, 31], enriched population of aldehyde dehydrogenase-bright stem cells (ALD-401) [4], neural stem/progenitor cells (NSPCs) [22] and bone marrow-derived mesenchymal stem cells (BM-MesSCs) [30], CD34+ hematopoietic stem/progenitor cells [24, 27], immature nerve cells and hemopoietic hepatic cells [21], LBS neurons [20], endothelial progenitor cells [17], MultiStem (HLM051) [14], olfactory ensheathing cells (OEC), and Schwann cells (SC) [22] (Table 3). Further details, such as administration route and administration window, are provided in supplementary material 2.
The most common route of cell administration was intravenous (15), and 6 studies have used intracerebral route for cell administration. In 5 studies, the cells were administered using intra-arterial route, 1 subarachnoid (spinal) and 1 intrathecal. The remaining studies used combinatorial approach (2).
The number of patients included in a specific type of trial and the distribution of the trials based on their design are shown in Table 4.
Effectiveness
National Institutes of Health Stroke Scale (NIHSS) score
We have evaluated available data from seven single-arm studies that reported the impact of stem cell therapies at 3-, 6-, and/or 12-month post-treatment. The NIHSS scores decreased in a modest manner after 3 (MD = −3.84 (95% CI −5.41 to −2.26)) and 6 (MD = −4.64 (95% CI −6.25 to −3.03)) months (Fig. 2a, b). In four studies, the change of NIHSS score was also available after 12 months post-treatment (MD = −3.19 (95% CI −4.74 to −1.63)) (Fig. 2c). The I2 scores were 85, 87, and 87%, respectively, indicating significant heterogeneity across the studies. NIHSS decrement in the studies having comparator arm was as follows: MD = −1.44 (95% CI −2.81 to −0.06), MD = −1.54 (95% CI −2.92 to −0.17), and MD = −2.19 (95% CI −3.95 to −0.43) after 3, 6, and 12 months, respectively (Fig. 2d, e, f). The studies had significant heterogeneity.
Barthel Index (BI) score
The BI change in follow-up studies without a comparator arm was as follows: MD = 18.19 (95% CI -5.63 to 42.00), MD = 22.24 (95% CI 4.35 to 40.12), and MD = 11.42 (95% CI 1.52 to 21.32) after 3, 6, and 12 months, respectively (Fig. 3a, b, c). studies (I score > = 77%). BI change in the studies having comparator arm was as follows: MD = 20.13 (95% CI 3.68 to 36.57), MD = 9.30 (95% CI 1.85 to 16.75), and MD = 8.08 (95% CI 3.14 to 13.01) after 3, 6, and 12 months, respectively (Fig. 3d, e, f). The studies had significant heterogeneity as in the previous case, however being moderate (I score = 62%) at 6-month evaluation.
Modified Rankin Scale (mRS) score
The mRS change in follow-up studies without a comparator arm was as follows: MD = −1.20 (95% CI −1.54 to −0.87), MD = −1.37 (95% CI −2.50 to −0.24), and MD = −1.80 (95% CI −3.42 to −0.18) after 3, 6, and 12 months, respectively (Fig. 4a, b, c). There was significant heterogeneity across studies (I score > = 92%). As for the studies with the comparator arm, the mRS change was as follows: MD = −0.03 (95% CI −0.77 to 0.71), MD = −0.20 (95% CI −0.83 to 0.44), and MD = −0.32 (95% CI −0.77 to 0.13), and I2 was 77 % and 57 % after 3, 6, and 12 months, respectively (Fig. 4d, e, f).
Risk of bias assessment
The allocation concealment was adequate in 7 studies, and random sequence generation was reported in 6 studies. Only two studies have used blinding of the participants and study personnel. There was only one case when the reviewers doubted the completeness of the outcomes in comparison with per-protocol data. The bias of other sources was hard to evaluate. The cumulative risk of bias table is shown on Fig. 5.
Adverse events
Seven cases of subdural and one extradural hematoma were observed which were assumed as related to the stem cell administration [4, 18, 37, 38]. The course was asymptomatic and without any neurological deterioration. One patient developed small infarct which was clinically silent [11]. In eight cases, the personnel observed a convulsion state among the patients, which was considered as non-related [4, 20, 38]. Five in control and 3 in experimental had seizures after stroke; 3 and 4 patients developed vascular recurrent stroke in control and experimental groups, respectively [28]. Two cases of recurrent stroke were observed [20, 33]. In the later, the relationship was considered as unclear. One case of serious deterioration in treatment group has been reported [34]. No other serious treatment-emergent adverse events were reported, or life-threatening adverse events and death were assessed to be not significantly different between the studies having comparator arm. No intracranial tumor formation during the study period was reported. Other events during the follow-up period were reported to be self-limiting and without any complications (supplementary material 2).
Discussion
The review evaluates up-to-date evidence of the usage of stem cell therapies for the improvement of clinical outcomes after stroke. Over the last 10 years, there is a strong interest in the potential of stem cells as a therapeutic modality after stroke. Although increasing in numbers, the studies lack a standardized approach, which makes it difficult to draw critical assumptions for the effectiveness of these therapies. Considering that the studies included in the analysis had substantial heterogeneity and variability in results, we have compared them based on different characteristics.
One of the most relevant parameters affecting the study outcome was the study design. As the non-comparator arm studies inherently lack a control arm, there is no baseline for comparison to ascertain whether the improvements were the consequence of the intervention or the result of standard care and brain repair mechanisms. The inclusion of a comparator arm, in contrast to a historical control or control to baseline, reduced the cases with significant improvement. As shown in Figs. 2b, 3b, and 4b, the improvement in NIHSS, BI, and mRS after 6 months in the studies without a comparator arm was observed in 11 out of 14 cases; however, the improvement of those parameters after 6 months was observed in only 5 out of 13 measurements in studies with a comparator arm (Figs. 2e, 3e, and 4e). Another observable point is that studies without a comparator arm show less improvement at 12 months when compared to 6 months (Figs. 2c, 3c, and 4c). This could be due to the fact of brain recovery at 12 months independent of the therapy. The evaluation at 3 months was not significantly different from those at 6 and 12 months, with the exception of the BI change, which was notably higher in studies featuring a comparator arm.
Interestingly, NIHSS reduction resulted in more significant improvement when compared to the cases when BI was implemented (Figs. 2 and 3). This can be explained by the different variables those tests measure. Whereas NIHSS checks neurological parameters like visual fields, facial palsy, and arms and legs drift, the BI focuses on functional parameters like independence in bathing, grooming, dressing, and toilet use. Consequently, NIHSS may be a more sensitive test in detecting improvements; however, those improvements may not be enough to significantly affect disability and functional recovery.
The studies with a comparator arm mainly used an intravenous injection of autologous stem cells with various outcomes. Nevertheless, one study with an intracerebral injection of autologous stem cells demonstrated improved outcomes both after 6 and 12 months [24]. On the other hand, the study with intra-arterial injection did not result in an improved outcome in any of the parameters.
We have analyzed studies with allogenic stem cells but none of them included a comparator arm. Therefore, we expect the results of upcoming clinical trials to see the effect of allogenic stem cells on restoration after stroke.
We also questioned why studies with a comparator arm using intravenous injection with autologous stem cells resulted in different outcomes (Fang et al. 2014-negative, Bang et al. 2005-positive, Prasad et al. 2014, Bhasin et al 2012, 2013-mixed). The difference may underlie in the study design, patient selection, stem cell preparation, and other variables.
The adverse effects reported in trials were self-limited or resolved after appropriate treatment. There was no alarming signal in relation to tumorigenicity and recurrent stroke. The reported adverse effects were mainly associated with the administration procedure, and the course was free of complications. The results, however, are self-limited and exhibit variability with respect to the duration of follow-up periods and the strategies employed for evaluation.
Future perspectives
Stem cell therapies act via multiple mechanisms, including the release of growth factors, anti-inflammatory effects, and possibly exosomes [18]. Although the regeneration mechanisms of the brain toward injury are still active long after stroke onset, they are insufficient to fully recover from damage. The synaptic plasticity changes, reorganization of existing neural circuits, and cell genesis, however, may be benefited from external stem cell administration. In our review, there is a non-significant trend toward improvement of some functional parameters included in the meta-analysis. However, the small extent of the improvement and the alarming level of heterogeneity across the studies arises a significant body of questions needed to be addressed in future clinical trials. The studies having comparator arm lacked a randomization approach per se, and the blinding procedure of the personnel and the study participants was missing in the majority of cases. In our opinion, such higher heterogeneity can be also due to a wide range of stem cell sources, administration route and window (supplementary materials 1 and 2). The time interval between the onset of stroke and cell therapy may be important in terms of the efficacy. When choosing the appropriate cell type and administration window, the majority of studies referred to previously reported findings on rodent models, although the extrapolation of these findings may not be always straightforward. Additionally, the optimal dose of stem cells is largely unknown. In this context, further studies should be conducted to understand the deep discrepancy between preclinical and clinical trials and execute phase 3 clinical trials with robust control of study characteristics and outcomes.
The review limitations include a relatively high percentage of small-size studies among others, a high extent of heterogeneity of stem cell usage, window and administration route, lack of opportunity to conduct subgroup analysis, and inherent obstacles coming from the non-availability of the additional information on request.
Data Availability
The data can be available upon reasonable request.
References
Benjamin EJ, Muntner P, Alonso A et al (2019) Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 139:e56–e528. https://doi.org/10.1161/CIR.0000000000000659
Laskowitz DT, Bennett ER, Durham RJ et al (2018) Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase 1 safety study. Stem Cells Transl Med 7:521. https://doi.org/10.1002/sctm.18-0008
Le ZX, Zhang XG, Huang YR et al (2021) Stem cell-based therapy for experimental ischemic stroke: a preclinical systematic review. Front Cell Neurosci 15:74. https://doi.org/10.3389/FNCEL.2021.628908/BIBTEX
Savitz SI, Yavagal D, Rappard G et al (2019) A phase 2 randomized, sham-controlled trial of internal carotid artery infusion of autologous bone marrow-derived ALD-401 cells in patients with recent stable ischemic stroke (RECOVER-Stroke). Circulation 139:192–205. https://doi.org/10.1161/CIRCULATIONAHA.117.030659
Marei HE, Hasan A, Rizzi R et al (2018) Potential of stem cell-based therapy for ischemic stroke. Front Neurol:9
Abdullahi AM, Abdullahi IM, Sarmast ST, Bhriguvanshi A (2021) Stem cell therapies for ischemic stroke: a systematic review. Cureus 13. https://doi.org/10.7759/CUREUS.13139
Kumar A, Rawat D, Prasad K (2021) Stem cell therapy in ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Ann Indian Acad Neurol 24:164. https://doi.org/10.4103/AIAN.AIAN_384_20
RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 1 Mar 2021
PRISMA. http://prisma-statement.org/PRISMAStatement/FlowDiagram. Accessed 3 Feb 2021
Bhasin A, Srivastava MV, Bhatia R et al (2012) Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med 8:181–189. https://doi.org/10.46582/jsrm.0803011
Prasad K, Mohanty S, Bhatia R et al (2012) Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: a pilot study. Indian J Med Res 136:221–228
Bhasin A, Padma Srivastava MV, Mohanty S et al (2016) Paracrine mechanisms of intravenous bone marrow-derived mononuclear stem cells in chronic ischemic stroke. Cerebrovasc Dis Extra 6:107–119. https://doi.org/10.1159/000446404
Tsang KS, Ng CPS, Zhu XL et al (2017) Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells 9:133–143. https://doi.org/10.4252/wjsc.v9.i8.133
Osanai T, Houkin K, Uchiyama S et al (2018) Treatment evaluation of acute stroke for using in regenerative cell elements (TREASURE) trial: rationale and design. Int J Stroke 13:444–448. https://doi.org/10.1177/1747493017743057
Deng L, Peng Q, Wang H et al (2019) Intrathecal injection of allogenic bone marrow-derived mesenchymal stromal cells in treatment of patients with severe ischemic stroke: study protocol for a randomized controlled observer-blinded trial. Transl Stroke Res 10:170–177. https://doi.org/10.1007/s12975-018-0634-y
Shichinohe H, Kawabori M, Iijima H et al (2017) Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): a study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol 17:179. https://doi.org/10.1186/s12883-017-0955-6
Fang J, Guo Y, Tan S et al (2019) Autologous endothelial progenitor cells transplantation for acute ischemic stroke: a 4-year follow-up study. Stem Cells Transl Med 8:14–21. https://doi.org/10.1002/sctm.18-0012
Levy ML, Crawford JR, Dib N et al (2019) Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke 50:2835–2841. https://doi.org/10.1161/STROKEAHA.119.026318
Vahidy FS, Haque ME, Rahbar MH et al (2019) Intravenous bone marrow mononuclear cells for acute ischemic stroke: safety, feasibility, and effect size from a phase I clinical trial. Stem Cells 37:1481–1491. https://doi.org/10.1002/stem.3080
Kondziolka D, Wechsler L, Goldstein S et al (2000) Transplantation of cultured human neuronal cells for patients with stroke. Neurology 55:565–569. https://doi.org/10.1212/WNL.55.4.565
Rabinovich SS, Seledtsov VI, Banul NV et al (2005) Cell therapy of brain stroke. Bull Exp Biol Med 139:126–128. https://doi.org/10.1007/s10517-005-0229-y
Chen L, Xi H, Huang H et al (2013) Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant 22. https://doi.org/10.3727/096368913X672154
Jiang Y, Zhu W, Zhu J et al (2013) Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant 22:2291–2298. https://doi.org/10.3727/096368912X658818
Chen DC, Lin SZ, Fan JR et al (2014) Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant 23:1599–1612. https://doi.org/10.3727/096368914X678562
Suárez-Monteagudo C, Hernández-Ramírez P, Álvarez-González L et al (2009) Autologous bone marrow stem cell neurotransplantation in stroke patients An open study. Restor Neurol Neurosci 27:151–161. https://doi.org/10.3233/RNN-2009-0483
Bang OY, Lee JS, Lee PH, Lee G (2005) Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57:874–882. https://doi.org/10.1002/ana.20501
Banerjee S, Bentley P, Hamady M et al (2014) Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med 3:1322–1330. https://doi.org/10.5966/sctm.2013-0178
Lee JS, Hong JM, Moon GJ et al (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28:1099–1106. https://doi.org/10.1002/stem.430
Honmou O, Houkin K, Matsunaga T et al (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134:1790–1807. https://doi.org/10.1093/brain/awr063
Qiao L-Y, Huang F-J, Zhao M et al (2014) A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant 23:65–72. https://doi.org/10.3727/096368914x684961
Laskowitz DT, Bennett ER, Durham RJ et al (2018) Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl Med 7:521–529. https://doi.org/10.1002/sctm.18-0008
Bhatia V, Gupta V, Khurana D, et al (2018) Randomized assessment of the safety and efficacy of intra-arterial infusion of autologous stem cells in subacute ischemic stroke. Am J Neuroradiol 39:899–904. https://doi.org/10.3174/ajnr.A5586
Taguchi A, Sakai C, Soma T et al (2015) Intravenous autologous bone marrow mononuclear cell transplantation for stroke: phase1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev 24:2207–2218. https://doi.org/10.1089/scd.2015.0160
Prasad K, Sharma A, Garg A et al (2014) Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke 45:3618–3624. https://doi.org/10.1161/STROKEAHA.114.007028
Hess DC, Wechsler LR, Clark WM et al (2017) Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 16:360–368. https://doi.org/10.1016/S1474-4422(17)30046-7
Bhasin A, Padma Srivastava MV, Mohanty S et al (2013) Stem cell therapy: a clinical trial of stroke. Clin Neurol Neurosurg 115:1003–1008. https://doi.org/10.1016/j.clineuro.2012.10.015
Kalladka D, Sinden J, Pollock K et al (2016) Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 388:787–796. https://doi.org/10.1016/S0140-6736(16)30513-X
Steinberg GK, Kondziolka D, Wechsler LR et al (2016) Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 47:1817–1824. https://doi.org/10.1161/STROKEAHA.116.012995
Ghali AA, Yousef MK, Ragab OAA, ElZamarany EA (2016) Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke patients. Front Neurol 7:225437. https://doi.org/10.3389/FNEUR.2016.00228/BIBTEX
Funding
Open access funding provided by University of Basel
Author information
Authors and Affiliations
Contributions
L. H., S. K, and S .M. performed the literature review. L. H., A. K, and S. M. wrote the main manuscript text. L. H. and S. M. prepared the figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hovhannisyan, L., Khachatryan, S., Khamperyan, A. et al. A review and meta-analysis of stem cell therapies in stroke patients: effectiveness and safety evaluation. Neurol Sci 45, 65–74 (2024). https://doi.org/10.1007/s10072-023-07032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07032-z