Abstract
Introduction
Patients with congenital heart disease (CHD) are at risk for cognitive and motor function impairments, brain injury, and smaller total brain volumes. The specific vulnerability of the cerebellum and its role in cognitive and motor functions in adults with congenital heart disease is not well defined.
Methods
Forty-three patients with CHD and 53 controls between 18 and 32 years underwent brain magnetic resonance imaging and cognitive, executive (EF), and motor function assessment. Cerebellar volumes were obtained using EasyMeasure and SUIT Toolbox. Associations between cerebellar volumes and cognitive and motor function were calculated using linear models.
Results
General cognitive and pure motor functions were lower in patients compared to controls (P < 0.05). Executive functions were within the normal range. While total cerebellar volumes and the anterior lobes were similar in patients and controls (P > 0.1), the posterior cerebellar lobe was smaller in patients with more complex CHD (P = 0.006). Smaller posterior cerebellar gray matter was not associated with cognitive functions. Smaller anterior cerebellar gray matter was not significantly related to motor functions (P > 0.1).
Conclusion
In adults with CHD, cerebellar volume was largely unimpaired. Patients with more complex CHD may be vulnerable to changes in the posterior cerebellar gray matter. We found no significant contribution of cerebellar gray matter to cognitive and motor impairments. More advanced imaging techniques are necessary to clarify the contribution of the cerebellum to cognitive and motor functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex CHD is a significant contributor to birth-defect-related morbidity [1, 2] and is associated with an increased risk of motor and neurocognitive function deficits [3]. In the 3rd trimester, CHD fetuses show a progressive decline in global brain growth, likely resulting from altered fetal circulation leading to abnormal oxygen and nutrient delivery [4, 5]. During this fetal period, the cerebellum is the region with the most rapid growth in healthy fetuses, suggesting a susceptibility of the cerebellum to hypoxic injury due to its high metabolic demand [6, 7]. One study found evidence of cerebral cortex dysmaturation within the microstructure in CHD neonates, where reduced cerebral oxygen delivery was related to impaired dendritic arborization, possibly making the cerebellum a particularly vulnerable structure to volumetric impairment as it is mainly comprised of gray matter [8]. In fact, smaller cerebellar volumes, along with smaller global brain volumes, have been previously observed in neonates, adolescents, and young adults with CHD [9,10,11]. However, the burden of cerebellar hypoplasia on neurodevelopmental outcomes remains mostly unclear.
It is well recognized that the cerebellum plays an important role in sensorimotor function, which is thought to be mainly associated with the anterior cerebellar lobe [12, 13]. In addition, current evidence indicates that the cerebellum plays a key role in cognitive and behavioral function. In particular, the posterior cerebellar lobe is thought to modulate cognitive processes via subcortical connections to the basal ganglia and the thalamus forming a cerebro-cerebellar loop [14], connections to the prefrontal cortex through the cerebello-thalamo-cortical and cortico-ponto-cerebellar pathways, passing through the superior and middle cerebellar peduncles [15, 16]. Functional magnetic resonance imaging (fMRI) studies describe activity in the posterior cerebellar lobules during cognitive tasks, such as working memory and inhibition, suggesting that the posterior cerebellum is engaged during a variety of executive function (EF) tasks as part of a functional network [14, 17,18,19].
Analysis of fMRI data from the Human Connectome Project including 787 subjects revealed cognitive task activation of lobule VI and VII, crus I II and lobule VIIB, IX, X of the cerebellum [20]. However, spatial compartmentalization of sensorimotor and cognitive function in the cerebellum may vary among individuals. One study found working memory and language tasks mainly activated regions in the posterior cerebellum (lobule VI and crus I). They found that activation patterns varied betwee individuals [18]. In addition, individual cerebellar areas are likely involved in more than one cognitive task, consistent with the modulating function of the cerebellum [18, 19].
Difficulties resulting from lesions in the posterior lobe are summarized in the cerebellar cognitive, affective syndrome and include executive function, spatial tasks, linguistic skills, and regulation of effect [19, 21]. The association of cognitive tasks with cerebellar volume has been investigated in a variety of studies and is likely age dependent [12, 14, 17]. In typically developing adolescents, higher scores on vocabulary, reading, working memory, and set-shifting were associated with increased gray matter volume in the posterior cerebellar lobe [12]. They found that the strength of the positive structure-function relationship of cerebellar gray matter and cognitive tasks was stronger with increasing age until adolescence. Another study found an association of cerebellar volume with working memory in children, but not in adults [14]. The cerebellum is involved in skills acquisition, which is particularly important during early childhood, and appears to be less important for the retention of learned behaviors [17]. Cerebellar recruitment may thus be more specialized and hence weaker in adulthood as tasks become more automated.
Preterm-born children comprise another population at risk for neurodevelopmental impairment, likely due to a vulnerability of the immature brain similar to the CHD population [22]. In preterm-born children, cerebellar injury and impaired cerebellar growth are frequently reported and associated with poor motor functions, poor cognitive and language functions, and behavioral problems consistent with the cerebellar cognitive, affective syndrome [23,24,25,26]. Smaller cerebellar volumes were associated with worse IQ in prematurely born adolescents. Similarly, an association of smaller cerebellar volume with worse motor performance was found in prematurely born children [23, 24]. Neurodevelopmental impairments in preterm-born and CHD population are similar, suggesting similar underlying structural impairments. In the preterm population, cerebellar volumetric impairment likely contributes to neurocognitive dysfunction. Similarly, the CHD population may be particularly susceptible to cerebellar hypoplasia due to impaired intrauterine growth, further highlighting the importance to study the cerebellum in the CHD population [27].
In CHD adults, information on the contribution of cerebellar volumes to executive impairments is rarely investigated, despite evidence suggesting that EF deficits persist into adulthood [28]. In CHD infants, perioperative cerebellar volume was associated with behavior regulation, fine motor function, and cognitive abilities [29]. Another study in adolescents with CHD found an association of cerebellar volume with a variety of executive function tasks, including working memory, cognitive flexibility, and inhibition [30]. In addition, cerebellar volume modulated the relationship between the prefrontal cortex and executive functioning [30]. One small study in CHD adults reported smaller volumes in the posterior cerebellum associated with poorer EF performance in the color-word-interference task, letter-number sequencing, matrix reasoning, and the BRIEF questionnaire and poorer motor performance in the grooved pegboard task [11]. However, Semmel and colleagues were not able to establish a link between the anterior cerebellum and motor function, measured with a pegboard task [11].
Therefore, the purpose of this study was to describe the burden of cerebellar volumetric alterations in the CHD population. Anterior and posterior lobes were analyzed separately to account for the spatial compartmentalization of the cerebellum, where the regions typically engaged during cognitive tasks are mainly located in the posterior cerebellar lobe [11, 12, 14, 17, 18]. We investigated the association of cerebellar gray matter with executive, motor function, processing speed, and the maintenance of attention in adults with CHD in comparison to healthy peers using a broad assessment battery of executive functions. We hypothesize that cerebellar gray matter is smaller in the CHD population and that smaller cerebellar volumes are associated with worse cognitive and motor function.
Methods
Study design
In this cross-sectional study, participants underwent neuropsychological assessments at the Zurich University Hospital and magnetic resonance brain imaging (MRI) at the University Children’s Hospital Zurich.
Study population
Young adults with CHD aged 18 to 32 years were recruited from two previous studies [31] between 2016 and 2018 at the University Hospital Zurich. Exclusion criteria for patients were as follows: genetic disorder, congenital or acquired neurological or psychiatric disorder affecting intellectual development (i.e. severe cerebral palsy, severe depression, or mental illness, preventing the patient from completing the neurocognitive assessment), or if patients were not fluent in the German language. Healthy controls matched to the adults with congenital heart disease (ACHD) were recruited as peers of the ACHD or through personal contacts. Controls were screened for comorbidities by means of a questionnaire and were excluded if they had any neurological or psychiatric condition. Of 67 ACHD, all underwent neuropsychological assessment and 46 underwent brain imaging (21 patients did not undergo MRI for the following reasons: MRI safety not ensured n = 12, claustrophobia n = 2, obesity n = 1, pregnancy n = 1, refused n = 5). The 21 patients who did not undergo MRI were not significantly different in disease complexity, presence of cyanosis, number of cardio-pulmonary bypass surgeries, age at first bypass surgery, and estimated IQ (P > 0.08).
Demographic variables
Demographic variables were collected through questionnaires: Years of education were measured as the number of school years until the completion of an initial education, which in Switzerland usually consists of 12 years of education for vocational training, 15 years for a bachelor’s degree, and 17 years for a master’s degree. Parental education was estimated based on maternal and paternal educational levels, using a 12-point scale ranging from 2 (lowest) to 12 (highest) parental education.
Cognitive function
Participants underwent comprehensive neurocognitive assessment, according to a test battery described previously [32].
IQ
Intelligence quotient (IQ) was estimated using a short form of the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV)[33].
Executive function
EF was assessed using an extensive test battery to capture the multidimensional and overlapping nature of executive functions [34, 35]. The following tests were included: the color-word-interference (CWI) test of the Delis-Kaplan Executive Function System (D-KEFS) [36] to assess inhibitory control and cognitive flexibility; the 5-Point Test [37] to evaluate nonverbal fluency; the S-word subtest of the Regensburger Wortflüssigkeitstest (RWT) [38] to assess verbal fluency; Standardized Link’s Probe (SLP) to assess goal-oriented action planning and constructive solution behavior [39]; Trail Making Test (TMT) to assess flexibility [40]; the computer-based stop signal task (SST) [41] was applied to measure response inhibition; the subtest verbal digit from the (WAIS-IV) [42] and visual block span (WMS-R) [43] were used to measure working memory. The Rey-Osterrieth Complex Figure (ROCF) was administered to measure visuomotor planning skills. This task requires participants to copy a complex figure on a blank paper as quickly and accurately as possible [44]. Test results were expressed as T-values according to the respective test manuals. From the T-values, an average was calculated to form a mean score for flexibility, inhibition, working memory, planning, visuomotor, and fluency to reduce the number of statistical tests performed. The following variables were used to form average scores: working memory: visual block span of the WMS-R and verbal digit span; inhibition: CWI and SST test; flexibility: CWI and TMT; planning: SLP and ROCF; fluency: 5-Point Test and the S-word subtest (RWT). For further details, see Supplemental Table 1.
Attention
An attention score was formed from the reaction times to visual and auditory presented stimuli of the Test of Attentional Performance Test [45].
Processing speed
A processing speed score was formed from the first condition of the color-word test [36], a measure for color processing speed, and the first condition of the trail making test, a measure for cognitive processing speed [40].
Neuromotor function
Pure and adaptive motor functions were assessed using the Zurich Neuromotor Assessment [46] from the time needed to fulfill the task. Pure motor functions include repetitive and alternating hand and foot movements as well as sequential finger movements. Adaptive motor functions were measured with a pegboard task. Results were expressed as z-scores.
Brain magnetic resonance imaging
Image acquisition
Participants underwent a research cerebral MRI scan on a 3T GE MR750 scanner. Hearing protection was provided with earplugs and headphones. For volumetric analysis, high-resolution three-dimensional (3D) T1-weighted images were acquired using a 3D spoiled gradient echo (SPGR) pulse sequence (TR = 11 ms, TE =5 ms, inversion time = 600 ms, FOV = 256 mm, matrix =256 × 192 mm, ST = 1 mm, flip-angle = 8°).
MRI processing
Total brain volume was obtained with FreeSurfer image analysis suite version 5.3.0, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). MRI images were then processed with EasyMeasure to obtain total cerebellar volumes and the SUIT toolbox to obtain cerebellar lobal volume. SUIT is a toolbox for the SPM (statistical parametric mapping) software used in Matlab. SUIT is developed to handle high inter-subject variability in the cerebellar anatomy. Preprocessing was done according to SUIT documentation, followed by isolation, normalization, and segmentation in the SUIT macro. Unlike the other segmentation methods, SUIT provides the volume of the individual cerebellar lobes, which can be added together to derive the volume of the cerebellar gray matter, but does not include the cerebellar white matter [11, 47]. To limit the number of statistical tests, we decided to combine lobules into anterior and posterior cerebellar gray matter instead of investigating single lobules. Total cerebellar volumes were obtained using the Cavalieri method of design-based stereology in combination with point counting using the EasyMeasure software. Stereological measurement of the cerebellum requires the determination of the number of test points that intersect the cerebellum using an array of probes overlaid onto the MR image and the slice thickness. For each slice, points are counted for each square grid of test points, overlaid on each image with a new random orientation of the square grid. The volume is computed as the sum of point count per section multiplied by the area covered by each test point and again multiplied by the sectioning interval. The grid size was selected to result in a coefficient of error (CE) below 5% [48]. The segmentation of 10 cases (5 per group) was performed twice by 2 raters (S.H. and N.N.) with an average percentage difference of 1.64% and mean coefficient of variation of 0.03. The remaining cases were segmented by one rater (S.H.).
Statistics
Descriptive statistics include mean and standard deviation (SD) or median and interquartile range (IQR) and frequencies. Group differences were calculated using the t-test and, for non-normal variables, the Mann-Whitney U test. The χ2 test was used for categorical variables. Non-normally distributed variables were log-transformed. Differences in cerebellar volumes between groups were calculated using linear regression, adjusted for total brain volume to account for global brain volumetric differences. The association of cerebellar volume with cognitive and motor functions was analyzed using linear regression with cognitive/motor function as the dependent variable and cerebellar volume as the independent variable. In the patient group only, the influence of the CHD category (simple, moderate, and complex) [49] on cerebellar volumes was analyzed using linear regression, adjusted for total brain volume. For regression models, estimates, standardized beta, 95% confidence interval (CI), and P-values of the effect are reported. Two-tailed P-values <0.05 were considered significant. In addition, the overall P-values of the model of the brain volumetric difference and association of cerebellar volume with cognitive and motor function were adjusted for multiple comparisons with FDR for three analyses separately (comparison of brain volumes, association of posterior cerebellum and cognitive function, association of anterior cerebellum and motor function) [50]. Analysis was conducted with the R software (R core team, version 3.4.2, URL https://www.Rproject.org/.).
Results
Study population
The study population was described previously [31]. A total of 46 patients and 54 controls underwent assessment of general cognitive ability, executive and motor function, and brain imaging. MRI processing with the SUIT toolbox and EasyMeasure was successful in 96 subjects. Thus, we report data of 43 ACHD (17 female) and 53 controls (26 female) assessed at a mean age of 26 years. ACHD and controls did not differ in sex and age (P > 0.1). Parental education was similar in ACHD and controls (8 (7.5; 10) vs. 9 (8; 10), P = 0.10). Median years of education were lower in ACHD than in controls (13 (12; 15) vs. 15 (14; 16), P = 0.010). All participants were of the Caucasian race. Cardiac diagnoses are listed in Supplemental Table 2. A total of 30 (69.8%) patients underwent at least one surgery requiring cardio-pulmonary bypass. Of these patients, 10 (21.3%) underwent more than one surgery on cardio-pulmonary bypass. Two subjects had a univentricular heart defect, and 12 (27.9%) had a repaired cyanotic defect. In the patient group, 15 patients had simple, 20 moderate, and 8 complex CHD. The median age at first bypass surgery was 1.38 (0.5; 6.2) years.
Subject outcome variables are presented in Table 1. IQ, attention, processing speed scores, and pure motor function were significantly lower in ACHD compared to controls (P < 0.05). However, EF functions were not different between the two groups (P > 0.1).
Comparison of cerebellar volumes between patients and controls
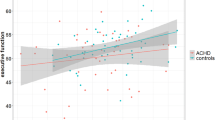
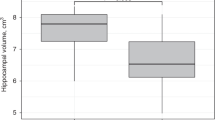
Total brain volume was smaller in patients compared to controls (B = −47.85, 95% CI: −90.8; −4.9, β = −0.22, P = 0.029); see Table 2. In contrast, total cerebellar volume was not different between patients and controls (B = 2.48, 95% CI: −2.1, 7.1, β = 0.088, P = 0.286, adjusted for total brain volume). When investigating cerebellar lobes separately, anterior cerebellar lobe and posterior cerebellar lobe were not different between patients and controls (anterior: B = −0.04, 95% CI: −0.6; 0.6, β = −0.01, P = 0.905; posterior: B = −1.52, 95% CI: −5.27, 2.24, β = −0.08, P = 0.425, adjusted for total brain volume). In the patient group, more complex CHD was associated with a smaller posterior cerebellar lobe (B = −4.3, 95% CI: −7.3, −1.2, β = −0.29, P = 0.006, overall model P < 0.001, adjusted for total brain volume); see Fig. 1. Similarly, there was a trend toward smaller anterior cerebellar lobe; however, the difference was not significant (B = −0.51, 95% CI: −1.05, 0.02, β = −0.19, P = 0.060, adjusted for total brain volume). There was no difference in total cerebellar volume depending on CHD complexity (B = −1.79, 95% CI: −6.5, 3.0, β = −0.08, P = 0.455). After adjusting for multiple comparisons, the posterior cerebellar lobe remained smaller in more complex CHD (P of the model <0.5).
Association of cerebellar lobes with cognitive and motor function
In Table 3, the association of cognitive, executive, and motor functions with the anterior or posterior cerebellar lobe is illustrated. In the combined group, the posterior cerebellar lobe was not associated with the IQ, attention, and processing speed score (P > 0.1). Smaller posterior cerebellar lobe was associated with worse inhibitory control, but not after adjusting for multiple comparisons (P of the model = 0.06). Similarly, the anterior cerebellar lobe was not associated with pure motor functions, and adaptive motor functions were measured using the pegboard task (P > 0.1). When exploring the group of patients with moderate and complex CHD separately in post hoc analyses, we did not find a significant association of EF and motor functions with cerebellar lobes (P > 0.05).
Discussion
In this current cohort of adults with CHD aged 18 to 32, we compared cerebellar volumes between patients and controls and explored the association of cerebellar volumes with executive and motor functions. Cerebellar volumes were similar between patients and controls after correcting for differences in total brain volume. Only the posterior cerebellar lobe was smaller in CHD compared to controls, in particular in patients with more complex CHD, and was associated with worse inhibitory control. We were not able to find an association of cerebellar volume with other EF, nor an association with motor functions.
General cognitive abilities and pure motor functions were significantly lower in ACHD compared to controls. However, ACHD performed within the normal range. Similar results have been previously described in numerous studies in CHD children, indicating only mild impairments in the CHD population present across several neurodevelopmental domains [52, 53], and increasing evidence suggests the persistence of EF and motor impairments in adults with CHD [28]. Of note, in our sample of adults with CHD, repetitive/alternating tapping movements were impaired, while there was no difference in the pegboard task compared to controls. This is in contrast to results from another study, reporting worse performance on the pegboard task in CHD adults compared to controls [11]. Differences in results may partially be attributed to differences in the patient population. The participants in the study by Semmel et al. had more severe CHD, with nearly half participants having a univentricular CHD diagnosis; all subjects underwent cardio-pulmonary bypass surgery, and prematurely born subjects >34 weeks of gestation were included. Consistent with the notion that motor impairments might be linked to smaller cerebellar volumes in severe CHD but not in more mild cases, another study found impaired psychomotor speed in adults with severe CHD, but not in the moderate CHD group [54]. Therefore, there is increasing evidence of persisting motor impairment beyond childhood in the CHD population, and healthcare professionals should consider the risk of motor dysfunction when counseling CHD adults.
In our cohort, total cerebellar volume and anterior cerebellar gray matter were similar in CHD patients and controls. However, in the patient group, the posterior cerebellar gray matter was smaller with increasing CHD complexity. This is in contrast to another study in CHD adults, where both anterior and posterior cerebellar gray matter volumes were reported to be smaller, although there were no differences in intracranial volume compared to healthy controls. However, in this previous study, total brain volume was not reported, and intracranial volume may poorly reflect total brain volume, as cerebrospinal fluid space may be enlarged in CHD patients with univentricular diagnosis [55]. Therefore, it is not known whether the cerebellar volume reductions reflect impaired global brain growth or whether cerebellar structures were particularly affected beyond the extent of total brain volume reductions. Of note, brain volumetric measures may be more affected by increasing CHD severity since posterior cerebellar volume reductions were more pronounced in our patients with complex CHD. Similarly, smaller brain volumes have been reported in adults with severe CHD, while brain volumes appear to be similar in adults with simple CHD and controls [56, 57]. Thus, patients with complex CHD may be particularly at risk for brain volume reductions.
We found an association of smaller posterior gray matter with worse inhibitory control, although this association did not survive FDR correction. This is consistent with another study in CHD adults and suggests that the cerebellum may contribute to inhibitory impairments in CHD adults [11]. We were not able to find an association between anterior or posterior cerebellar volumes and IQ, attention or processing speed, and most EF. This result stands in contrast to the association between posterior cerebellar volumes and executive functioning reported by Semmel et al. [11], although, consistent with the Semmel et al. study, we observed that anterior cerebellar volume was not associated with motor function impairments. In the preterm population, an association of the cerebellum with motor impairments has been previously reported [24]. While the preterm and CHD population show similar risk for neurodevelopmental impairment [58], the cerebellum may be more vulnerable in the preterm population, where cerebellar injury is considered a common complication of preterm birth [25]. Furthermore, cerebellar involvement in executive and motor dysfunction is complex and likely varies among individuals [18]. Although we tried to account for spatial compartmentalization of the cerebellum by investigating anterior and posterior cerebellar gray matter separately, we were not able to account for more individualized differences. It has been previously suggested that a cerebro-cerebellar loop could mediate EF performance [17], and thus, cerebellar volumes may poorly reflect cerebellar function and more advanced imaging techniques, such as diffusion tensor imaging to extract brain tracts, which may be more sensitive to involvement of the cerebellum in EF. In addition, lab-based tasks to assess EF skills usually involve several brain regions; for example, the color-word-inference task activates brain regions in the frontal, parietal, and occipital regions, yet research often investigates associations of EF with single brain regions [59]. Furthermore, participants may use different strategies to solve the same task, thus involving different brain regions [60]. Recently, the concept of separable EF domains has been challenged, with EF likely involving partly overlapping cognitive functions [61]. Due to these challenges, we used a large test battery to comprehensively assess EF in our ACHD participants.
Our study has some limitations. We were not able to investigate the effect of prematurity on cerebellar volume since gestational age was not collected as part of this study. In addition, we were not able to investigate the effect of a specific cardiac diagnosis on EF as we included a variety of CHD, although by including a range of cardiac diagnoses, our sample was more representative of the CHD general population. Our comprehensive test battery may have resulted in selecting mostly high-functioning CHD adults (IQ 97.5). Thus, detecting differences in cognitive and executive functioning may have been more difficult due to only mild impairments. However, it is important to study the participants with mild CHD to better understand the mechanism of neurocognitive impairment in the general CHD population.
In conclusion, cerebellar volumes were mostly unimpaired in our CHD cohort. The only structure smaller in CHD was the posterior cerebellar lobe, in particular in patients with complex CHD. This finding indicates an increased risk of cerebellar abnormalities in patients with complex CHD. We found weak evidence for an association between cerebellar posterior gray matter and worse inhibitory control. Other EF did not correlate with the cerebellar volumes, suggesting minor or more complex involvement of the cerebellum in EF. Larger studies with more advanced imaging techniques are necessary to further clarify the involvement of the cerebellum in EF.
Data availability
The de-identified data are available from the corresponding author upon reasonable request.
Abbreviations
- ACHD:
-
adults with congenital heart disease
- CHD:
-
congenital heart disease
- CI:
-
confidence interval
- EF:
-
executive function
- IQ:
-
intelligence quotient
- IQR:
-
interquartile range
- MRI:
-
magnetic resonance imaging
- SD:
-
standard deviation
- SE:
-
standard error
References
Jenkins KJ, Botto LD, Correa A et al (2019) Public health approach to improve outcomes for congenital heart disease across the life span. J Am Heart Assoc 8:e009450. https://doi.org/10.1161/JAHA.118.009450
Marino BS, Lipkin PH, Newburger JW et al (2012) Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 126:1143–1172. https://doi.org/10.1161/CIR.0b013e318265ee8a
Latal B (2016) Neurodevelopmental Outcomes of the child with congenital heart disease. Clin Perinatol 43:173–185. https://doi.org/10.1016/j.clp.2015.11.012
Limperopoulos C, Tworetzky W, McElhinney DB et al (2010) Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121:26–33. https://doi.org/10.1161/CIRCULATIONAHA.109.865568
Peyvandi S, Latal B, Miller SP et al (2019) The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage 185:776–782. https://doi.org/10.1016/j.neuroimage.2018.05.045
Andescavage NN, du Plessis A, McCarter R et al (2017) Complex trajectories of brain development in the healthy human fetus. Cereb Cortex 27:5274–5283. https://doi.org/10.1093/cercor/bhw306
Owen M, Shevell M, Donofrio M et al (2014) Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr 164:1121–1127.e1121. https://doi.org/10.1016/j.jpeds.2013.11.033
Kelly CJ, Christiaens D, Batalle D et al (2019) Abnormal microstructural development of the cerebral cortex in neonates with congenital heart disease is associated with impaired cerebral oxygen delivery. J Am Heart Assoc 8:e009893. https://doi.org/10.1161/JAHA.118.009893
Meuwly E, Feldmann M, Knirsch W et al (2019) Postoperative brain volumes are associated with one-year neurodevelopmental outcome in children with severe congenital heart disease. Sci Rep 9:10885. https://doi.org/10.1038/s41598-019-47328-9
Bolduc ME, Lambert H, Ganeshamoorthy S et al (2018) Structural brain abnormalities in adolescents and young adults with congenital heart defect: a systematic review. Dev Med Child Neurol 60:1209–1224. https://doi.org/10.1111/dmcn.13975
Semmel ES, Dotson VM, Burns TG et al (2018) Posterior cerebellar volume and executive function in young adults with congenital heart disease. J Int Neuropsychol Soc 24:939–948. https://doi.org/10.1017/S1355617718000310
Moore DM, D'Mello AM, McGrath LM et al (2017) The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Dev Cogn Neurosci 24:1–11. https://doi.org/10.1016/j.dcn.2016.12.001
Konczak J, Timmann D (2007) The effect of damage to the cerebellum on sensorimotor and cognitive function in children and adolescents. Neurosci Biobehav Rev 31:1101–1113. https://doi.org/10.1016/j.neubiorev.2007.04.014
Clark SV, Semmel ES, Aleksonis HA et al (2021) Cerebellar-subcortical-cortical systems as modulators of cognitive functions. Neuropsychol Rev. https://doi.org/10.1007/s11065-020-09465-1
Beez T, Munoz-Bendix C, Steiger HJ et al. Functional tracts of the cerebellum-essentials for the neurosurgeon. Neurosurg Rev 2021; 44: 273-278. https://doi.org/10.1007/s10143-020-01242-1
Palesi F, De Rinaldis A, Castellazzi G et al (2017) Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep 7:12841. https://doi.org/10.1038/s41598-017-13079-8
Stoodley CJ, Limperopoulos C (2016) Structure-function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med 21:356–364. https://doi.org/10.1016/j.siny.2016.04.010
Ashida R et al (2019) Sensorimotor, language, and working memory representation within the human cerebellum. Human Brain Map 40(16):4732–4747. https://doi.org/10.1002/hbm.24733
Silveri MC (2021) Contribution of the cerebellum and the basal ganglia to language production: speech, word fluency, and sentence construction—evidence from pathology. The Cerebellum 20(2):282–294. https://doi.org/10.1007/s12311-020-01207-6
Guell X, Gabrieli JD, Schmahmann JD (2018) Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172:437–449. https://doi.org/10.1016/j.neuroimage.2018.01.082
Schmahmann JD (2018) The cerebellum and cognition. Neurosci Lett. https://doi.org/10.1016/j.neulet.2018.07.005
Morton PD, Ishibashi N, Jonas RA (2017) Neurodevelopmental abnormalities and congenital heart disease: insights into altered brain maturation. Circ Res 120:960–977. https://doi.org/10.1161/CIRCRESAHA.116.309048
Matthews LG, Inder TE, Pascoe L et al (2018) Longitudinal preterm cerebellar volume: perinatal and neurodevelopmental outcome associations. Cerebellum 17:610–627. https://doi.org/10.1007/s12311-018-0946-1
Brossard-Racine M, du Plessis AJ, Limperopoulos C (2015) Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum 14:151–164. https://doi.org/10.1007/s12311-014-0597-9
Hortensius LM, Dijkshoorn ABC, Ecury-Goossen GM et al (2018) Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics:142. https://doi.org/10.1542/peds.2018-0609
Limperopoulos C, Bassan H, Gauvreau K et al (2007) Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120:584–593. https://doi.org/10.1542/peds.2007-1041
Wehrle FM et al (2022) Similarities and differences in the neurodevelopmental outcome of children with congenital heart disease and children born very preterm at school entry. J Pediatr. https://doi.org/10.1016/jpeds.2022.05.047
Keir M, Ebert P, Kovacs AH et al (2019) Neurocognition in adult congenital heart disease: how to monitor and prevent progressive decline. Can J Cardiol 35:1675–1685. https://doi.org/10.1016/j.cjca.2019.06.020
Brossard-Racine M, Panigrahy A (2022) Structural brain alterations and their associations with function in children, adolescents, and young adults with congenital heart disease. Canad J Cardiol 39(2):123–132. https://doi.org/10.1016/j.cjca.2022.10.028
Badaly D, Beers SR, Ceschin R, Lee VK, Sulaiman S, Zahner A et al (2022) Cerebellar and prefrontal structures associated with executive functioning in pediatric patients with congenital heart defects. Front Neurol 13:827780. https://doi.org/10.3389/fneur.2022.827780
Naef N, Schlosser L, Brugger P et al (2021) Brain volumes in adults with congenital heart disease correlate with executive function abilities. Brain Imaging Behav. https://doi.org/10.1007/s11682-020-00424-1
Schlosser L, Kessler N, Feldmann M et al (2022) Neurocognitive functioning in young adults with congenital heart disease: insights from a case-control study. Cardiol Young 32:694–701. https://doi.org/10.1017/S1047951121002705
Daseking M, Petermann F, Waldmann H-C (2014) Schätzung der allgemeinen Intelligenz mit einer Kurzform der WAIS-IV bei neurologischen Fragestellungen. Aktuelle Neurologie 41:349–355
Baggetta P, Alexander PA (2016) Conceptualization and operationalization of executive function. Mind, Brain, Ed 10:10–33
Miyake A, Friedman NP (2012) The nature and organization of individual differences in executive functions: four general conclusions. Curr Dir Psychol Sci 21:8–14. https://doi.org/10.1177/0963721411429458
Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive function system: examiners manual. Psychol Corp
Regard M, Strauss E, Knapp P (1982) Children’s production on verbal and non-verbal fluency tasks. Percep Motor Skills 55:839–844
Aschenbrenner S, Tucha O, Lange K. Regensburger Wortflüssigkeitstest Hogrefe Göttingen. In: Germany; 2000:
Metzler P (2000) Standardisierte Link'sche Probe-SLP-Manual zur Beurteilung exekutiver Funktionen. Swets & Zeitlinger, Frankfurt AM
Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Reitan. Neuropsychology
Verbruggen F, Logan GD, Stevens MA (2008) STOP-IT: Windows executable software for the stop-signal paradigm. Behav Res Methods 40:479–483
Petermann F (2012) Wechsler Adult Intelligence Scale (WAIS-IV; deutsche Version). Pearson, Frankfurt
Härting C. Wechsler-Gedächtnistest: WMS-R; Deutsche Adaption der revidierten Fassung der Wechsler Memory Scale. Huber; 2000
Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique.(Les problems.). Archives de psychologie 1941
Zimmermann P, Fimm B. Testbatterie zur Erfassung von Aufmerksamkeitsstörungen (TAP 2.3.). In: Psytest; 2012
Largo RH, Caflisch JA, Hug F et al (2001) Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol 43:436–443
Diedrichsen J (2006) A spatially unbiased atlas template of the human cerebellum. Neuroimage 33:127–138. https://doi.org/10.1016/j.neuroimage.2006.05.056
Doherty CP, Fitzsimons M, Holohan T et al (2000) Accuracy and validity of stereology as a quantitative method for assessment of human temporal lobe volumes acquired by magnetic resonance imaging. Magn Reson Imaging 18:1017–1025. https://doi.org/10.1016/s0730-725x(00)00185-5
Warnes CA, Liberthson R, Danielson GK et al (2001) Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 37:1170–1175
Cao J, Zhang S (2014) Multiple comparison procedures. JAMA 312:543–544. https://doi.org/10.1001/jama.2014.9440
Cohen J (1988) Statistical power analysis for the behavioral sciences. Routledge, Abingdon England
Latal B, Wohlrab G, Brotschi B et al (2016) Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J Pediatr. https://doi.org/10.1016/j.jpeds.2016.06.050
Mills R, McCusker CG, Tennyson C et al (2018) Neuropsychological outcomes in CHD beyond childhood: a meta-analysis. Cardiol Young 28:421–431. https://doi.org/10.1017/S104795111700230X
Klouda L, Franklin WJ, Saraf A et al (2017) Neurocognitive and executive functioning in adult survivors of congenital heart disease. Congenit Heart Dis 12:91–98. https://doi.org/10.1111/chd.12409
Heye KN, Knirsch W, Latal B et al (2018) Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion. Pediatr Res 83:63–70. https://doi.org/10.1038/pr.2017.203
Asschenfeldt B, Evald L, Heiberg J et al (2020) Neuropsychological status and structural brain imaging in adults with simple congenital heart defects closed in childhood. J Am Heart Assoc 9:e015843. https://doi.org/10.1161/JAHA.120.015843
Cordina R, Grieve S, Barnett M et al (2014) Brain volumetric, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. Neuroimage Clin 4:319–325. https://doi.org/10.1016/j.nicl.2013.12.011
Easson K, Dahan-Oliel N, Rohlicek C et al (2019) A comparison of developmental outcomes of adolescent neonatal intensive care unit survivors born with a congenital heart defect or born preterm. J Pediatr 207:34–41.e32. https://doi.org/10.1016/j.jpeds.2018.11.002
Yeo BT, Krienen FM, Eickhoff SB et al (2015) Functional specialization and flexibility in human association cortex. Cereb Cortex 25:3654–3672. https://doi.org/10.1093/cercor/bhu217
Pietschnig J, Penke L, Wicherts JM et al (2015) Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci Biobehav Rev 57:411–432. https://doi.org/10.1016/j.neubiorev.2015.09.017
Doebel S (2020) Rethinking executive function and its development. Perspect Psychol Sci 15:942–956. https://doi.org/10.1177/1745691620904771
Acknowledgements
We would like to thank all the adults who participated in this study.
Funding
Open access funding provided by University of Zurich This project is supported by the Swiss Heart Foundation, the Mäxi Foundation, and the Olga-Mayenfisch Foundation.
Author information
Authors and Affiliations
Contributions
N.N. wrote the manuscript and performed the statistical analysis. N.N., S.H., and R.T. performed the imaging analysis with different tools. B.L., M.G., and R.T. developed the design of the study. L.S. performed the neuropsychological assessment and MR imaging. All co-authors critically reviewed the manuscript. Non-author contributors are Peter Brugger and the Children’s Heart and Development Research Group.
Corresponding author
Ethics declarations
Ethical approval
The ethics committee of the Canton of Zurich in Switzerland approved this study, and written informed consent was obtained from all participants. This study was performed in line with the principles of the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Reporting guidelines checklist
The STROBE checklist has been applied.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 13 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naef, N., Hottinger, S.J., Schlosser, L. et al. Association of cerebellar volume with cognitive and motor function in adults with congenital heart disease. Neurol Sci 44, 3979–3987 (2023). https://doi.org/10.1007/s10072-023-06861-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06861-2