Abstract
Introduction
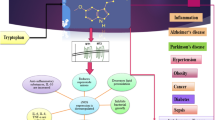
Insomnia is a common condition that may be caused by or coexist with other medical or psychological illnesses. Nearly a quarter of a billion people across the globe suffer from insomnia frequently. Lemborexant, a dual orexin receptor antagonist, is a recently authorized hypnotic-based medication for insomnia. The purpose of this systematic review is to further investigate its efficacy and safety profile, with the primary goal of comparing the effects of two FDA-approved doses of lemborexant, 5 mg and 10 mg (LEM5 and LEM10, respectively).
Materials and methods
PubMed, Google Scholar, ClinicalTrials.gov, and Cochrane Central were searched for relevant literature, and studies were considered if they compared the efficacy and safety of lemborexant 5 mg to lemborexant 10 mg. This study comprised clinical trials.
Results
A total of 6 studies were evaluated for efficacy and safety of lemborexant therapy. They reported a significant betterment in values pertaining to sleep efficacy, sleep onset latency, wake after sleep onset, total sleep time, sleep quality, ISI score, and morning alertness. The results presented a dose-dependent pattern and showed slight variation with the different dosages. The most prevalent side effects noted were somnolence, headaches, and dizziness, with infections like UTIs and upper respiratory tract infections also being commonly reported. The incidence is rather ambiguous and not sincerely dose-dependent. The differences between results for LEM5 and LEM10 do not exhibit a wide variation, although slight dose-dependent alterations are noted.
Conclusion
Lemborexant is well integrated with the amelioration of sleep disturbances in insomniac patients, as shown by a decrease in eSOL and sWASO and a rise in sSE, sTST, quality of sleep, and morning alertness. Effects last 12 months after therapy.

Similar content being viewed by others
Change history
18 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10072-023-06626-x
References
Sateia MJ (2014) International classification of sleep disorders-third edition. Chest. 146(5):1387–1394
45 insomnia statistics: how many people suffer from insomnia? https://www.thegoodbody.com/insomnia-statistics/
Fang H, Tu S, Sheng J, Shao A (2019) Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 23(4):2324–2332
Jaussent I, Bouyer J, Ancelin ML, Akbaraly T, Peres K, Ritchie K et al (2011) Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 34(8):1103–1110
Jarrin DC, Alvaro PK, Bouchard MA, Jarrin SD, Drake CL, Morin CM (2018) Insomnia and hypertension: a systematic review. Sleep Med Rev. 41:3–38
Taylor DJ, Lichstein KL, Durrence HH (2003) Insomnia as a health risk factor. Behav Sleep Med 1(4):227–247
Cunnington D, Junge MF, Fernando AT (2013) Insomnia: prevalence, consequences and effective treatment. Med J Aust 199(S8)
Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH et al (2018) Sleep and cognitive decline: a prospective nondemented elderly cohort study. Ann Neurol. 83(3):472–482
Gureje O, Kola L, Ademola A, Olley BO (2009) Profile, comorbidity and impact of insomnia in the Ibadan study of ageing. Int J Geriatr Psychiatry. 24(7):686–693
Unsal P, Sengul Aycicek G, Deniz O, Esme M, Dikmeer A, Balcı C et al (2021) Insomnia and falls in older adults: are they linked to executive dysfunction? Psychogeriatrics. 21(3):359–367
Roach M, Juday T, Tuly R, Chou JW, Jena AB, Doghramji PP (2021) Challenges and opportunities in insomnia disorder. Int J Neurosci 131(11):1058–1065
Sivertsen B, Øverland S, Bjorvatn B, Mæland JG, Mykletun A (2009) Does insomnia predict sick leave? J Psychosom Res. 66(1):67–74
Bollu PC, Kaur H Sleep medicine: insomnia and sleep. Mo Med 116(1):68–75
Taddei-Allen P (2020) Economic burden and managed care considerations for the treatment of insomnia. Am J Manag Care 26(4):S91–S96
Edinger JD, Arnedt JT, Bertisch SM, Carney CE, Harrington JJ, Lichstein KL et al (2021) Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 17(2):255–262. https://doi.org/10.5664/jcsm.8986
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG et al (2017) European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 26(6):675–700
Abad VC, Guilleminault C (2018) Insomnia in elderly patients: recommendations for pharmacological management. Drugs & Aging 35(9):791–817. https://doi.org/10.1007/s40266-018-0569-8
Pizza F, Barateau L, Dauvilliers Y, Plazzi G (2022) The orexin story, sleep and sleep disturbances. J Sleep Res 31(4). https://pubmed.ncbi.nlm.nih.gov/35698789/
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN (2017) Adverse effects of hypnotic medications. J Clin Sleep Med 13(6):839 PMC5443747
Equihua AC, de La Herrán-Arita AK, Drucker-Colin R (2013) Orexin receptor antagonists as therapeutic agents for insomnia. Front Pharmacol. 163
Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K et al (2012) Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology 79(23):2265–2274. https://pubmed.ncbi.nlm.nih.gov/23197752/
Roth T, Black J, Cluydts R, Charef P, Cavallaro M, Kramer F et al (2017 Feb) Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep 40(2). https://pubmed.ncbi.nlm.nih.gov/28364509/
Connor KM, Mahoney E, Jackson S, Hutzelmann J, Zhao X, Jia N et al (2016) A phase II dose-ranging study evaluating the efficacy and safety of the orexin receptor antagonist filorexant (MK-6096) in patients with primary insomnia. Int J Neuropsychopharmacol 19(8):1–10 PMC5006195/
Murphy P, Moline M, Mayleben D, Rosenberg R, Zammit G, Pinner K et al (2017) Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med 13(11):1289–1299. https://pubmed.ncbi.nlm.nih.gov/29065953/
Onge ES, Phillips B, Rowe C (2022) Daridorexant: a new dual orexin receptor antagonist for insomnia. J Pharm Technol 38(5):297–303. https://doi.org/10.1177/87551225221112546
DORAs for insomnia. https://www.healthline.com/health/insomnia/insomnia-treatment-targets-wakefulness
Xue T, Wu X, Chen S, Yang Y, Yan Z, Song Z et al (2022) The efficacy and safety of dual orexin receptor antagonists in primary insomnia: a systematic review and network meta-analysis. Sleep Med Rev 61. https://pubmed.ncbi.nlm.nih.gov/34902823/
Kishi T, Nomura I, Matsuda Y, Sakuma K, Okuya M, Ikuta T et al (2020) Lemborexant vs suvorexant for insomnia: a systematic review and network meta-analysis. J Psychiatr Res 128:68–74. https://pubmed.ncbi.nlm.nih.gov/32531478/
U.S. FDA approves Eisai’s DAYVIGOTM (lemborexant) for treatment of insomnia in adult patients | News Release:2019 | Eisai Co., Ltd. 2022. https://www.eisai.com/news/2019/news201993.html
McElroy H, O’Leary B, Adena M, Campbell R, Monfared AAT, Meier G (2021) Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis. J Manag Care Spec Pharm [Internet]. 27(9):1296–1308. https://pubmed.ncbi.nlm.nih.gov/34121443/
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372. https://pubmed.ncbi.nlm.nih.gov/33782057/
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343(7829). https://www.bmj.com/content/343/bmj.d5928
Study to evaluate the effect of 2 dosage strengths of lemborexant (E2006) on a multiple sleep latency test in participants with insomnia disorder - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02350309
Rosenberg R, Murphy P, Zammit G, Mayleben D, Kumar D, Dhadda S et al (2019) Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open 2(12). https://pubmed.ncbi.nlm.nih.gov/31880796/
Kärppä M, Yardley J, Pinner K, Filippov G, Zammit G, Moline M et al (2020) Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep 43(9):1–11. https://pubmed.ncbi.nlm.nih.gov/32585700/
Yardley J, Kärppä M, Inoue Y, Pinner K, Perdomo C, Ishikawa K et al (2021) Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep Med 80:333–342. https://pubmed.ncbi.nlm.nih.gov/33636648/
Roth T, Rosenberg R, Morin CM, Yardley J, Pinner K, Perdomo C et al (2022) Impact of lemborexant treatment on insomnia severity: analyses from a 12-month study of adults with insomnia disorder. Sleep Med 90:249–257. https://pubmed.ncbi.nlm.nih.gov/35220140/
Grandner MA (2017) Sleep, health, and society. Sleep Med Clin 12(1):1–22. https://pubmed.ncbi.nlm.nih.gov/28159089/
Fuller PM, Gooley JJ, Saper CB (2006) Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 21(6):482–493
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG et al (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18(23):9996 PMC6793310/
Nixon JP, Kotz CM, Novak CM, Billington CJ, Teske JA (2012) Neuropeptides controlling energy balance: orexins and neuromedins. Handb Exp Pharmacol 209(209):77 PMC4736749
Janto K, Prichard JR, Pusalavidyasagar S (2018) An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med. 14(8):1399 PMC6086961/
Richardson GS, Zammit G, Wang-Weigand S, Zhang J (2009) Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry 70(4):18843. https://www.psychiatrist.com/jcp/neurologic/neurology/safety-subjective-sleep-effects-ramelteon-administration
Clark JW, Brian ML, Drummond SPA, Hoyer D, Jacobson LH (2020) Effects of orexin receptor antagonism on human sleep architecture: a systematic review. Sleep Med Rev 53. https://pubmed.ncbi.nlm.nih.gov/32505969/
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341. https://pubmed.ncbi.nlm.nih.gov/20171303/
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The above article was published with error. Figure 1 (PRISMA Flowchart) has been replaced with the incorrect figure during correction stage.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habiba, U., Waseem, R., Shaikh, T.G. et al. Comparative efficacy and safety of lemborexant 5 mg versus 10 mg for the treatment of insomnia: a systematic review. Neurol Sci 44, 1533–1541 (2023). https://doi.org/10.1007/s10072-023-06601-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06601-6