Abstract
Introduction
We aimed to clarify the differences in static and dynamic diaphragm parameters between the expiratory and inspiratory phases in amyotrophic lateral sclerosis (ALS).
Methods
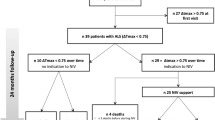
Twenty patients with early-stage ALS and 16 healthy controls were enrolled in the study. We measured the amplitudes of compound muscle action potential (phCMAP) by electrical stimulation of the phrenic nerve and the zone of apposition wall thickness of the diaphragm (DT) using ultrasonography. We analyzed the differences in phCMAP (∆phCMAP) and DT (∆DT) between the end-inspiratory and end-expiratory phases and their correlation with forced vital capacity (FVC).
Results
The ΔphCMAP (mean 129.7 ± SD 204.7 µV) and ∆DT (0.80 ± 0.88 cm) in patients were significantly smaller than those in controls (348.6 ± 247.7 µV, p = 0.0003 and 1.89 ± 1.10 cm, p = 0.0002, respectively). Although ∆DT was significantly correlated with FVC, we found no correlation between ∆phCMAP and FVC. The phCMAP was paradoxically smaller during inspiration than during expiration in 35% of patients but in none of the controls.
Conclusion
Dynamic parameters of the diaphragm were abnormal in early-stage ALS. The paradoxical reduction in phCMAP during inspiration may reflect early respiratory dysfunction. Assessment of dynamic abnormalities of the diaphragm may provide helpful information for respiratory management in patients with ALS.






Similar content being viewed by others
References
Sarwal A, Walker FO, Cartwright MS (2013) Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 47:319–329. https://doi.org/10.1002/mus.23671
Matsuda C, Shimizu T, Nakayama Y, Haraguchi M (2019) Cough peak flow decline rate predicts survival in patients with amyotrophic lateral sclerosis. Muscle Nerve 59:168–173. https://doi.org/10.1002/mus.26320
de Carvalho M, Swash M, Pinto S (2019) Diaphragmatic neurophysiology and Respiratory Markers in ALS. Front Neurol 10:143. https://doi.org/10.3389/fneur.2019.00143
Lechtzin N, Cudkowicz ME, de Carvalho M, Genge A, Hardiman O, Mitsumoto H, Mora JS et al (2018) Respiratory measures in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 19:321–330. https://doi.org/10.1080/21678421.2018.1452945
Andersen PM, Borasio GD, Dengler R, Hardiman O, Kollewe K, Leigh PN, Pladat PF et al (2005) EFNS task force on management of amyotrophic lateral sclerosis: guidelines for diagnosing and clinical care of patients and relatives. Eur J Neurol 12:921–938. https://doi.org/10.1111/j.1468-1331.2011.03501.x
Bolton CF (1993) AAEM minimonograph #40: clinical neurophysiology of the respiratory system. Muscle Nerve 16:809–818. https://doi.org/10.1002/mus.880160802
Evangelista T, Carvalho M, Pinto A, Luís ML (1995) Phrenic nerve conduction in amyotrophic lateral sclerosis. J Neurol Sci 129:35–37. https://doi.org/10.1016/0022-510x(95)00057-9
Pinto S, Geraldes R, Vaz N, Pinto A, de Carvalho M (2009) Changes of the phrenic nerve motor response in amyotrophic lateral sclerosis: longitudinal study. Clin Neurophysiol 120:2082–2085. https://doi.org/10.1016/j.clinph.2009.08.025
Pinto S, Turkman A, Pinto A, Swash M, de Carvalho M (2009) Predicting respiratory insufficiency in amyotrophic lateral sclerosis: the role of phrenic nerve studies. Clin Neurophysiol 120:941–946. https://doi.org/10.1016/j.clinph.2009.02.170
Pinto S, Pinto A, de Carvalho M (2012) Phrenic nerve studies predict survival in amyotrophic lateral sclerosis. Clin Neurophysiol 123:2454–2459. https://doi.org/10.1016/j.clinph.2012.05.012
de Carvalho M, Pinto S, Swash M (2018) Diaphragm motor responses to phrenic nerve stimulation in ALS: surface and needle recordings. Clin Neurophysiol 129:349–353. https://doi.org/10.1016/j.clinph.2017.11.019
Miranda B, Gromicho M, Pereira M, Pinto S, Swash M, de Carvalho M (2020) Diaphragmatic CMAP amplitude from phrenic nerve stimulation predicts functional decline in ALS. J Neurol 267:2123–2129. https://doi.org/10.1007/s00415-020-09818-z
de Carvalho MD. Electrodiagnostic assessment of respiratory dysfunction in motor neuron disease. In:. Handbook of Clinical Neurophysiology BV 2004 Eisen A, editor. 2004 (Chapter 30):513–28
Laghi FA Jr, Saad M, Shaikh H (2021) Ultrasound and non-ultrasound imaging techniques in the assessment of diaphragmatic dysfunction. BMC Pulm Med 21:85. https://doi.org/10.1186/s12890-021-01441-6
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD (2008) Monitoring recovery from diaphragm paralysis with ultrasound. Chest 133:737–743. https://doi.org/10.1378/chest.07-2200
Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T (2016) Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve 53:522–527. https://doi.org/10.1002/mus.24902
Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M (2016) Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol 127:892–897. https://doi.org/10.1016/j.clinph.2015.03.024
Yoshioka Y, Ohwada A, Sekiya M, Takahashi F, Ueki J, Fukuchi Y (2007) Ultrasonographic evaluation of the diaphragm in patients with amyotrophic lateral sclerosis. Respirology 12:304–307. https://doi.org/10.1111/j.1440-1843.2006.01029.x
Aliberti S, Messinesi G, Gramegna A, Tremolizzo L, Susani E, Pesci A (2013) Diaphragm ultrasonography in the management of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 14:154–156. https://doi.org/10.3109/21678421.2012.762931
Hiwatani Y, Sakata M, Miwa H (2013) Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener 14:127–131. https://doi.org/10.3109/17482968.2012.729595
de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K et al (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–503. https://doi.org/10.1016/j.clinph.2007.09.143
Hobson-Webb LD, Simmons Z (2019) Ultrasound in the diagnosis and monitoring of amyotrophic lateral sclerosis: a review. Muscle Nerve 60:114–123. https://doi.org/10.1002/mus.26487
Ueki J, De Bruin PF, Pride NB (1995) In vivo assessment of diaphragm contraction by ultrasound in normal subjects. Thorax 50:1157–1161. https://doi.org/10.1136/thx.50.11.1157
Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 6:8. https://doi.org/10.1186/2036-7902-6-8
Theerawit P, Eksombatchai D, Sutherasan Y, Suwatanapongched T, Kiatboonsri C, Kiatboonsri S (2018) Diaphragmatic parameters by ultrasonography for predicting weaning outcomes. BMC Pulm Med 18:175. https://doi.org/10.1186/s12890-018-0739-9
Chen R, Collins S, Remtulla H, Parkes A, Bolton CF (1995) Phrenic nerve conduction study in normal subjects. Muscle Nerve 18:330–335. https://doi.org/10.1002/mus.880180311
Resman-Gaspersc A, Podnar S (2008) Phrenic nerve conduction studies: technical aspects and normative data. Muscle Nerve 37:36–41. https://doi.org/10.1002/mus.20887
Hadjikoutis S, Wiles CM (2001) Respiratory complications related to bulbar dysfunction in motor neuron disease. Acta Neurol Scand 103:207–213
Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155:1570–1574. https://doi.org/10.1164/ajrccm.155.5.9154859
Lloyd T, Tang YM, Benson MD, King S (2006) Diaphragmatic paralysis: the use of M mode ultrasound for diagnosis in adults. Spinal Cord 44:505–508. https://doi.org/10.1038/sj.sc.3101889
Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, Brochard L (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38:796–803. https://doi.org/10.1007/s00134-012-2547-7
DiNino E, Gartman EJ, Sethi JM, McCool FD (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69:423–427. https://doi.org/10.1136/thoraxjnl-2013-204111
Fantini R, Tonelli R, Castaniere I, Tabbì L, Pellegrino MR, Cerri S, Livrieri F et al (2019) Serial ultrasound assessment of diaphragmatic function and clinical outcome in patients with amyotrophic lateral sclerosis. BMC Pulm Med 19:160. https://doi.org/10.1186/s12890-019-0924-5
Sartucci F, Pelagatti A, Santin M, Bocci T, Dolciotti C, Bongioanni P (2019) Diaphragm ultrasonography in amyotrophic lateral sclerosis: a diagnostic tool to assess ventilatory dysfunction and disease severity. Neurol Sci 40:2065–2071. https://doi.org/10.1007/s10072-019-03938-9
Ottenheijm CA, Heunks LM, Dekhuijzen RP (2008) Diaphragm adaptations in patients with COPD. Respir Res 9:12. https://doi.org/10.1186/1465-9921-9-12
Shimizu T, Komori T, Kugio Y, Fujimaki Y, Oyanagi K, Hayashi H (2010) Electrophysiological assessment of corticorespiratory pathway function in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 11:57–62. https://doi.org/10.1080/17482960903207385
Acknowledgements
The authors thank Kiyomi Koike, Kohko Yoshimoto, Noriko Murayama, Kazuma Shinozuka, and Sachiko Kaneko from the Division of Neurophysiology, Tokyo Metropolitan Neurological Hospital for providing technical support.
Author information
Authors and Affiliations
Contributions
RM and TS designed this study. RM, YI, HK, and KB performed the nerve conduction study and ultrasonography. RM and TS analyzed the data and drafted the manuscript. KT and MI supervised the study and approved the final manuscript for publication. All authors have approved the final article.
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Ethical approval
The study was approved by the Ethics Committee of Tokyo Metropolitan Neurological Hospital (TS-H29-004).
Informed consent
All participants provided written informed consent for the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10072_2022_6371_MOESM1_ESM.pdf
Supplementary file1 Fig. S1. Heatmap showing p-values between parameters by Pearson's correlation coefficient in patients with ALS. SVC: slow vital capacity, FVC: forced vital capacity, phCMAPe: the amplitudes of phCMAP at end-expiratory phases, phCMAPi: the amplitudes of phCMAP at end-inspiratory phases, ΔphCMAP: difference between phCMAPe and phCMAPi, phCMAP ratio: phCMAPi/phCMAPe, CMAP fraction: 100 x (phCMAPi – phCMAPe)/(phCMAPe), DTe: DT at end-expiratory phases, DTi: DT at end-inspiratory phases, ΔDT: difference between DTe and DTi, DT ratio: DTi/DTe, TFdi: 100 x (phCMAPi-phCMAPe)/(phCMAPe), Vi: the maximum velocity of the diaphragm in M-mode during normal inhalation, Vf: the maximum velocity of the diaphragm in M-mode during forced inhalation, Vs: the maximum velocity of the diaphragm in M-mode during sniffing. Fig. S2: Heatmap showing R-values between parameters by Pearson's correlation coefficient in patients with ALS. SVC: slow vital capacity, FVC: forced vital capacity, phCMAPe: the amplitudes of phCMAP at end-expiratory phases, phCMAPi: the amplitudes of phCMAP at end-inspiratory phases, ΔphCMAP: difference between phCMAPe and phCMAPi, phCMAP ratio: phCMAPi/phCMAPe, CMAP fraction: 100 x (phCMAPi – phCMAPe)/(phCMAPe), DTe: DT at end-expiratory phases, DTi: DT at end-inspiratory phases, ΔDT: difference between DTe and DTi, DT ratio: DTi/DTe, TFdi: 100 x (phCMAPi-phCMAPe)/(phCMAPe), Vi: the maximum velocity of the diaphragm in M-mode during normal inhalation, Vf: the maximum velocity of the diaphragm in M-mode during forced inhalation, Vs: the maximum velocity of the diaphragm in M-mode during sniffing. Fig. S3. Correlations between slow vital capacity (SVC, %) and phrenic nerve compound muscle action potential (phCMAP) in patients with amyotrophic lateral sclerosis using Pearson’s correlation coefficient. (A) phCMAPe: the amplitudes of phCMAP at end-expiratory phases. (B) phCMAPi: the amplitudes of phCMAP at end-inspiratory phases. (C) ΔphCMAP: difference between phCMAPe and phCMAPi. (D) phCMAP ratio: phCMAPi/phCMAPe. (E) CMAP fraction: 100 x (phCMAPi – phCMAPe)/(phCMAPe). Fig. S4. Correlations between slow vital capacity (SVC, %) and diaphragm thickness (DT) parameters in patients with amyotrophic lateral sclerosis using Pearson’s correlation coefficient. (A) DTe: DT at end-expiratory phases. (B) DTi: DT at end-inspiratory phases. (C) ΔDT: difference between DTe and DTi. (D) DT ratio: DTi/DTe. (E) TFdi: 100 x (phCMAPi-phCMAPe)/(phCMAPe). Fig. S5. Correlations between slow vital capacity (FVC, %) and parameters of dome movement velocity using Pearson’s correlation coefficient. (A) Vi: the maximum velocity of the diaphragm in M-mode during normal inhalation, (B) Vf: the maximum velocity of the diaphragm in M-mode during forced inhalation, (C) Vs: the maximum velocity of the diaphragm in M-mode during sniffing (PDF 169 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morishima, R., Shimizu, T., Ishizaka, Y. et al. The difference in the diaphragmatic physiological measures between inspiratory and expiratory phases in ALS. Neurol Sci 43, 6821–6830 (2022). https://doi.org/10.1007/s10072-022-06371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06371-7