Abstract
Objective
To investigate the potential detection rate of anti-thyroid antibodies’ (ATAbs) positivity, thyroid dysfunctions, and autoimmune thyroid diseases (AITDs) in autoimmune encephalitis (AE) and to analyze whether thyroid autoimmunity/dysfunction can affect the clinical course of AE.
Methods
Two hundred twenty-one AE patients and 229 age- and sex-matched controls were included in this study. We measured the levels of ATAbs (anti-thyroglobulin antibodies [TgAb], anti-thyroid peroxidase anti-bodies [TPOAb]) and thyroid hormones in all the individuals. In addition, the association of thyroid autoimmunity/dysfunctions with functional outcomes of AE was identified by using logistic regression and Kaplan–Meier analyses.
Results
The prevalence of TPOAb-positive and TgAb-positive was significantly higher in AE patients (16.3% and 16.7%, respectively) as compared with controls (9.6% and 7.4%, respectively; P = 0.034 and P = 0.002, respectively). In addition, the free triiodothyronine (fT3) level was significantly lower in AE patients as compared to the controls (P < 0.001). However, the frequency of AITDs (Hashimoto’s thyroiditis and Graves’ disease) did not significantly differ between AE patients and control subjects. Importantly, low fT3 was found to be associated with poor functional outcomes at the 3-month follow-up in AE. Adjustment of potential confounders did not change the association. However, the presence of ATAbs did not significantly alert the disease course of AE.
Conclusions
ATAbs-positive and/or AITD patients with symptomatic encephalopathy should undergo proper surveillance for AE. Moreover, low fT3 could serve as a possible predictor of poor short-term outcome in AE, thereby suggesting that monitoring of thyroid function in AE may be necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune encephalitis (AE) associated with antibodies against cell surface or synaptic proteins is a group of inflammatory brain disorders [1]. Before the discovery of N-methyl-D-aspartate receptor (NMDAR) antibodies in 2007 [2], patients with AE could be misdiagnosed with Hashimoto encephalopathy (HE) [3]. HE still remains a controversial condition, which has been defined as steroid-responsive encephalitis and associated with anti-thyroid antibodies (ATAbs) [4]. In several case reports, the patients with positive ATAbs and diagnosed with suspected HE that later turned out to be cell surface antibody–positive AE have been described [5]. It has been reported that during the acute stage, both AE and HE share similar clinical presentations, such as seizures, memory deficits, and psychiatric symptoms. However, HE and neuronal antibody–positive AE are considered as different disorders. The differential diagnosis of neuronal antibody-positive AE and HE is clinically important because AE is caused by dysfunction of neurons due to interactions of neuronal receptors with antibodies, which can be potentially triggered by tumors or abnormal immune conditions. In addition, patients with HE had been found to respond well to the steroids. For patients with AE, their immune treatment methods may be varied. The first-line treatment includes steroid intravenous immunoglobulins [IVIG] and plasma exchange. The second-line treatment includes cyclophosphamide, rituximab, and mycophenolate mofetil. HE still remains a controversial entity in the twenty-first century. Until now, no neuronal antibody associated with HE was found. The high serum levels of TPOAb-associated neurological syndrome had been found due to a completely unrelated etiology [1]. An early diagnosis and immunotherapy can contribute to better outcome of AE patients [6]. According to the diagnostic criteria of 2016, the diagnosis of HE should be conclusively made only after exclusion of AE and other syndromes associated with defined auto-antibodies [1].
A number of previous studies have demonstrated that thyroid dysfunction may have an influence on the poor clinical outcomes in some diseases (such as stroke, traumatic brain injury, or encephalitis) [7,8,9], but there are only few studies that have previously focused on the relationship between the thyroid dysfunction and the functional outcome of AE. Moreover, autoimmune thyroid diseases (AITDs) are the most commonly diagnosed organ-specific autoimmune diseases, which mainly includes Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) [10]. ATAbs, such as anti-thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TgAb), are generally considered as sensitive serum markers of AITDs [11]. An increased prevalence of ATAbs and AITDs have been found in several autoimmune diseases such as myasthenia gravis [12], neuromyelitis optical spectrum disorder [13], and multiple sclerosis [14]. Patients with AE are observed to have a high frequency of positive ATAbs in several previous reports, although the numbers of AE cases investigated were relatively small [15, 16]. However, so far, to the best of our knowledge, no study has previously described detailed clinical characteristics of patients with both AE and AITDs.
Thus, we aimed to evaluate the possible detection rate of ATAbs positivity as well as thyroid dysfunctions in patients with AE and also to investigate whether thyroid autoimmunity/dysfunction can significantly affect the clinical course of AE. In addition, the clinical presentations, laboratory findings, temporal patterns, treatment, and prognosis of patients with coexistence of AE and AITDs have been also analyzed.
Methods
Patients
In this observational study, patients with definite AE and who visited the neurology center, West China Hospital, from June 2012 to September 2020 were retrospectively reviewed. AE was diagnosed according to the 2016 diagnostic criteria as described in our previous study [1, 17]. Exclusion criteria used were as follows: (1) patients diagnosed with infectious encephalitis, toxic metabolic encephalopathy, cerebral malaria, brain abscess, brain tumor, prion diseases, or unknown cause encephalitis; (2) patients who did not test the serum samples for ATAbs and thyroid function tests; and (3) patients with missing data. The control group included subjects who visited our hospital for health examination.
Clinical evaluation
The data related with the demographics, clinical features, the results of auxiliary examinations, treatment modalities, outcomes, and clinical relapses were collected by experienced neurologists. Brain magnetic resonance imaging (MRI) results were systematically assessed by radiologists and neurologists. Routine electroencephalogram (EEG) results were evaluated by experienced neurophysiologists. The modified Rankin scale (mRS) was used to evaluate the functional outcome of AE [18]. All the data in a unified report form were collected from the hospital medical records or the database of the One-WC (Outcomes of anti-NMDAR Encephalitis Study in Western China; registration number: ChiCTR1800019762) registry study.
Laboratory testing
The fasting blood samples were collected at 6–7 a.m. of the second day after admission, including hemoglobin (Hb) levels, neutrophil-to-lymphocyte ratio (NLR), total cholesterol, uric acid, and plasma fibrinogen. In addition, the testing for serum thyroid function levels during the first admission (before immunotherapy) included serum free triiodothyronine (fT3, reference range, 3.60–7.50 pmol/l), free thyroxine (fT4, reference range, 12.0–22.0 pmol/l), thyrotropin (TSH, reference range, 0.27–4.20 mU/l), TPOAb (reference range, 0–34 U/ml), and TgAb (reference range, 0–115 U/ml), and these were tested by a Roche immunoanalyzer (Moduler EE, E170D) [19].
Cerebrospinal fluid (CSF) and serum samples of all patients were screened prior to administering immunotherapy. The antibodies panel included NMDAR, anti-LGI-1, anti-CASPR2, anti-GABAAR, anti-GABABR, anti-AMPA1, anti-AMPA2, anti-DPPX, anti-lgLON5. An evaluation of antibodies was performed according to the previously reported studies [17, 20].
Follow-up and outcome
In this study, the mRS score was evaluated by follow-up clinic visits from the patients or/and their caregivers every 3 months after disease onset. The follow-up period was at least 3 months after the disease onset for all patients. The primary outcome was a poor outcome at the 3-month follow-up. The secondary outcomes included relapse and death at the last follow-up.
Definition
As previously reported, overt hypothyroidism was defined by increased TSH, and a decrease in fT4, whereas subclinical hypothyroidism was defined by raised TSH and normal level of fT4. Overt hyperthyroidism was defined by decrease in TSH and increased fT4 and/or fT3, and subclinical hyperthyroidism was defined by decreased TSH and normal level of fT4 and fT3 [21]. HT was defined by the high level of TPOAb, raised TSH, and/or thyroid ultrasonography (hypoechogenic appearance) [22]. Furthermore, the diagnosis of GD was based on the clinical symptoms, low TSH with elevated fT4 and/or fT3 levels, positive TRAb and/or thyroid ultrasonography (diffuse goiter, and decreased as well as dishomogeneous echogenicity) [23]. Thyroid ultrasonography was not included in the routine examination.
The relapse of AE was identified as a new onset or worsening of the symptoms after at least 2 months of stabilization or initial improvement [24]. A mRS score of > 2 was defined as a poor functional outcome.
Statistical analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) version 25.0 (SPSS Inc., Chicago, IL, USA) and R software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). In this study, continuous variables were presented as median (range) or mean ± SD; and categorical variables were presented as an absolute number (percentage). We applied the Mann–Whitney U test for continuous variables with non-normal distribution and t test for continuous variables with normal distribution. Chi-square test or Fisher’s exact test were used to calculate the categorical variables. A two-sided P value < 0.05 was considered statistically significant. Bonferroni correction was used for multiple testing. Multiple logistic regression was used to examine the association of thyroid function with functional outcome at the 3-month follow-up.
Results
Demographics and clinical features of patients with AE and controls
A total of 221 patients with AE (mean age: 34.5 ± 15.58 years; female/male: 128:93) were included in this study. Among them, 156 patients with NMDAR antibodies, 41 patients with LGI1 antibodies and/or CASPR2 antibodies, and 24 patients with GABABR antibodies were detected. The demographical and clinical features of the patients with AE and controls have been summarized in Table 1. These controls also matched the patients with AE for sex and age.
Comparison of detection rate of ATAbs-positive and thyroid dysfunction in patients with AE and the controls
As shown in Table 1, compared to the controls, patients with AE presented a significantly higher ATAbs (19.9% vs 12.2%, P = 0.026), TPOAb (16.3% vs 9.6%, P = 0.034) and TgAb seroprevalence rate (16.7% vs 7.4%, P = 0.002). The TSH level was significantly higher in patients with AE than in the controls (P < 0.001), while the fT3 level was significantly lower in patients with AE than in controls (P < 0.001). In addition, in patients with AE, 87.3% were euthyroid, 7.7% had subclinical hypothyroidism, 0.9% had overt hyperthyroidism, 2.3% had subclinical hyperthyroidism, and 1.8% had overt hypothyroidism. Moreover, there was no significant difference in the frequency of thyroid dysfunction between the two groups. Finally, compared to the controls, patients with AE displayed a higher frequency of AITDs, although this difference was not found to be statistically significant (3.1% vs 1.7%; P = 0.381).
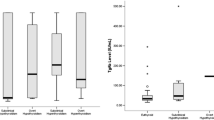
With regard to different types of AE, the proportion of patients with ATAbs-positive was observed to be highest in the GABABR group (41.7%) followed by the LGI1/CASPR2 group (31.7%) and the NMDAR group (13.5%). TgAb- and TPOAb-positive rates were also noted to be statistically different among these groups (P < 0.05; Fig. 1).
A comparison of the frequency of ATAbs-positive in the different types of autoimmune encephalitis. The detection rate of ATAbs positivity was found to be highest in the GABABR group followed by the LGI1/CASPR2 group and the NMDAR group. The ATAbs-positive rate was statistically different among these groups (P < 0.05). ATAbs = anti-thyroid antibodies; CASPR2 = contactin associated protein-2; GABABR = gamma-aminobutyric-acid receptor; LGI1 = leucine-rich glioma-inactivated protein-1; NMDAR = N-methyl-D-aspartate receptor. Blue bars represent the number of patients with ATAbs positivity. Red line represents the prevalence of ATAbs positivity of the different types of autoimmune encephalitis
Association of the clinical course of AE with ATAbs-positivity and thyroid dysfunction
Our data showed that all of the 44 AE patients with ATAbs-positive presented seizures (37/44, 84.1%), behavior dysfunction (40/44, 91.0%), cognitive deficits (30/44, 68.1%), movement disorders (16/44, 36.4%), disturbance of consciousness (19/44, 43.2%), speech disturbance (16/44, 36.4%), autonomic dysfunction (23/44, 52.3%), central hypoventilation (7/44, 16.0%), and sleep disturbance (20/44, 45.5%). As shown in Tables 2 and 3, based on the univariate analysis, compared to AE patients with ATAbs-negative, age at onset was higher in AE patients who were detected with ATAbs-positive (P < 0.05). The proportion of elevated antinuclear antibody (ANA) was significantly higher in the ATAbs-positive group (P < 0.05). All patients with both ATAbs- and ANA-positive had an ANA positive titer of at least 1:100. Our data also showed that NLR was significantly higher in the TPOAb-positive group as compared to the TPOAb-negative group (P = 0.03). Moreover, AE patients with TgAb-positive had significantly higher levels of total cholesterol as compared with TgAb-negative AE patients (P = 0.023). However, when the AE patients with ATAbs-positive were compared with those with ATAbs-negative, significant difference was not observed in the sex, clinical symptoms, the proportion of tumors, abnormal EEG, and abnormal brain MRI. In addition, univariate analysis also indicated that the disturbance of the consciousness (P = 0.002), Hb (P = 0.01), and NLR (p = 0.005) were significantly different between the low fT3 and non-low fT3 groups.
Overall, 34.8% (77/221) of patients with the poor outcome (mRS score > 2) were diagnosed at the 3-month follow-up. Univariate analysis showed that low fT3 could be a significant predictive value (P = 0.001; odds ratio, 0.6; 95% confidence interval, 0.442–0.813) for poor outcome at the 3-month follow-up. This association remained significant even in the multivariate model that was adjusted for age, sex, systolic blood pressure, disturbance of consciousness, Hb, total cholesterol, and NLR (Fig. 2). However, no statistical significance between poor functional outcome and fT4, TSH, TPOAb, and TgAb was observed in this study.
Multiple analyses between the thyroid function and poor outcome at the 3-month follow-up in AE. Because of relatively small numbers of outcomes, model 1 was adjusted for sex and age. Model 2 was adjusted for systolic blood pressure, disturbance of consciousness, hemoglobin, total cholesterol, and neutrophil-to-lymphocyte ratio in addition to model 1. AE = autoimmune encephalitis; CI = confidence interval; fT3 = free triiodothyronine; fT4 = free thyroxin; OR = odds ratio; TgAb = anti-thyroglobulin antibodies; TPOAb = anti-thyroid peroxidase antibodies; TSH = thyrotropin
During a median follow-up of 24 months (range, 3–90 months), a total of 40 (18.1%) patients experienced clinical relapses. The median interval from onset to the first relapse was approximately 6 months (range: 2–54). Kaplan–Meier curve demonstrated a higher trend of clinical relapses in patients with ATAbs-positive, but these were found to be not statistically significant (TPOAb-positive vs TPOAb -negative, 25.0 vs 16.8% P = 0.16; TgAb-positive vs TgAb -negative, 27.0 vs 16.3% P = 0.1). In addition, during the follow-up period, 15 patients (6.8%) died with a median interval of 6 months from the symptom onset to the death (range, 2.5–21 months). Kaplan–Meier curve showed a similar probability of survival rate among the different thyroid function groups (Supplementary Fig. 1).
Clinical characteristics of patients with AE and AITDs
Of these 221 patients, seven patients were found to have evidence of AITDs, including HT (n = 5) and GD (n = 2). Other autoimmune diseases were found in 3 of 221 patients, including one with psoriasis (0.5%), one with systemic lupus erythematosus (0.5%), and one with Sjogren’s syndrome (0.5%). Supplementary Table 1 shows the detailed clinical characteristics of the patients with AE and AITDs, and all of these patients did not have any other autoimmune disorders.
Among the five AE patients with HT, the onset of these two different diseases was found to be approximately concomitant. Three HT patients who also exhibited overt hypothyroidism were treated with synthetic levothyroxine therapy. Two patients (patient 2 and 5) were diagnosed GD prior to AE, and the time between the onset of both the conditions were 4 and 2 years, respectively. Three patients received only IVIG and four patients received IVIG and MP treatment. In addition, four patients received levothyroxine (L-T4) treatment, one patient received thiamazole treatment, and two patients without treatment for AITDs. One GD patient (patient 2) had hypothyroid exposure to radioactive iodine-131 and maintained L-T4 treatments daily. And one GD patient (patient 5) received substitution with thiamazole when AE was diagnosed. Since only a small number of patients were diagnosed with AITDs, the exact comparison of AE patients with and without AITDs could not be carried out.
Discussion
The present report describes a comprehensive ATAbs (TPOAb and TgAb) and thyroid function profile (TSH, fT4 and fT3) among a cohort of AE patients. It was observed that compared to sex- and age-matched controls, AE patients with significantly higher frequencies of TPOAb-positive and TgAb-positive, higher TSH levels. and lower fT3 levels. In addition, low fT3 level served as a significant predictive value for poor outcome at the 3-month follow-up in AE patients. However, the presence of ATAbs did not significantly alter the disease course of AE. Finally, the prevalence of patients with AE that co-existed with other AITDs was also identified and their clinical features were described.
A few previously published studies with small sample size found that ATAbs-positivity could be detected in 30–50% AE patients [15, 16]. In this study, although a relatively low prevalence of ATAbs-positive in patients with AE was observed, our results are in accordance with previous studies that reported a significantly higher incidence of ATAbs-positivity in AE as compared to controls. Moreover, the proportion of patients with ATAbs-positivity was higher in those with anti-LGI1/CASPR2 encephalitis and anti-GABABR encephalitis as compared to those with anti-NMDAR encephalitis. Given that a significant higher percentage of AE patients show laboratory evidence of ATAbs-positivity, our findings suggest that when a patient might be suspected of having HE, antibodies against neural cell-surface should also be screened to make a differential diagnosis.
Lin et al. reported that in patients with anti-NMDAR encephalitis, the ATAbs-positive group had a higher occurrence of epileptic seizures and conscious disturbances, as well as higher mRS scores [16]. However, our data showed that clinical characteristics and prognosis of AE was almost similar in both the ATAbs-positive and ATAbs-negative groups. It is possible that such differences may be a consequence of small sample size. Previous studies have also speculated that the presence of ATAbs-positivity may not be directly pathogenic and it may cause immune dysfunction in the brain by promoting cross-reaction with antibodies against the cell-surface or synaptic proteins [3]. Interestingly, although the difference was not statistically significant, relapse was more common in patients with ATAbs-positivity than in those with ATAbs-negativity. Thus, whether ATAbs-positivity plays a role in the relapse of AE remains to be explored.
The present study also focused on the thyroid function profiles among AE patients. We found that AE patients had lower fT3 and higher TSH levels as compared to the controls. Previously, thyroid hormone abnormalities have been observed in various medical conditions [7,8,9, 25]. Importantly, our data indicated that low fT3 levels upon admission were associated with relatively poor short-term functional outcomes in AE patients, which was consistent with prior reports related to the short-term prognostic value of low fT3 in some diseases including encephalitis [9], stroke [26], severe brain injury [8], and coronavirus infections 2019 [25]. However, we report for the first time that a significantly down-regulated fT3 level was observed in AE, which suggest that AE may trigger other immune pathways. One possible explanation may be that systemic inflammation can reduce deiodinase activity thereby converting total thyroxine to total triiodothyronine, leading to low fT3 levels [25]. The correlation of low fT3 and NLR in this study further supported the role of systemic inflammation although detailed mechanisms remain to be elucidated. Further prospective studies with a larger sample size are warranted to verify the potential prognostic implications of the low fT3 in AE. Additionally, anti-TSH receptor antibodies were not included in the routine examination because all patients enrolled did not have a history of any symptoms of hyperthyroidism except of the two GD patients. The absence of data on anti-TSH receptor antibodies could be considered as a weakness of this study. However, it is not very essential for our research, which has mainly presented the findings related to autoimmunity in AE patients.
To date, the coexistence of AE and AITDs has been described only rarely (Supplementary Table 2). Binks S et al. indicated that patients with LGI1 and CASPR2 antibodies may often display coexisting AITDs (8/68 [11.8%] and 1/31 [3.2%], respectively) [27]. Zhao et al. demonstrated that 5.4% of patients with AE had HT [28], which is consistent with our results (3.1%). Although our data showed that the overall prognosis of patients with AE and AITDs was favorable, the results have to be interpreted with great caution as the size of patients used was quite small. Importantly, 5 patients with AE were complicated with HT. The mechanism of the two diseases coexist at the same time remains unclear. Although these patients had symptoms of encephalitis and elevated TPOAb, they should not be diagnosed with HE according to the 2016 diagnostic criteria, which suggested that AE should be considered in the presence of neuronal antibodies [1]. It has been reported that TPOAb-carriers are more likely to carry neuronal antibodies compared to TPOAb-negative individuals [29]. The increased TPOAb may reflect a subclinical HT and that the associated neurologic syndrome is due to a completely unrelated etiology [3]. Moreover, our study showed that in two patients, GD onset was prior to AE onset. To our knowledge, only four cases of coincident GD and AE have been reported previously [29,30,31,32]. Given the increased risk of concurrent GD in patients with AE, it therefore suggested that, when GD patients display symptomatic encephalopathy, a comprehensive diagnostic evaluation should be strongly encouraged.
The relationship between AE and AITDs has been incompletely understood so far. The coexistence of AE and AITDs may result from genetic predisposition and/or different immunological factors. Firstly, previous studies have shown that HLA class II alleles can be associated with immune-mediated conditions (including anti-NMDAR encephalitis, anti-LGI1 encephalitis and GD) [30, 31]. Thus, genetic predisposition may have played a significant role in the development of both the diseases. In addition, T cells recently have been suggested to play a vital role in immunopathogenic mechanisms thus triggering both AE and AITDs [32, 33]. Therefore, it can be speculated that the T cells may contribute to the coexistence of both AE and AITD by diverse mechanisms. However, the detailed mechanisms underlying the coexistence of AE and AITDs remain to be explored in the future. Furthermore, in our cohort, three patients with AE also have other autoimmune diseases, such as psoriasis, systemic lupus erythematosus, and Sjogren’s syndrome. And the prevalence of elevated ANA was significantly higher in the ATAbs-positive AE group. The presence of ANA illustrates probably the autoimmune background, which may lead to the development of AE.
There are some limitations in this study. First, the results of this study should be interpreted cautiously due to its retrospective design. Second, seven patients with both AITD and AE were diagnosed in our study. Their CNS clinical presentation was not different as compared to the patients with AE only. The exact comparison of AE patients with and without AITDs could not be carried out due to relatively small number of patients with AITD and AE in this cohort. Further studies are needed to clarify these important aspects.
Conclusions
In conclusion, a high rate of ATAbs-positivity was found in AE, and AITDs may be comorbidities associated with AE. Thus, ATAbs-positive and/or AITDs patients with symptomatic encephalopathy should be informed about the possible diagnosis of AE. Furthermore, low fT3 was identified as an independent predictor of poor short-term functional outcomes for AE. The thyroid function profiles might thus provide valuable information for carefully assessing the risk of poor functional outcomes in AE patients.
Data availability
All data contained in the article and the datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Graus F, Titulaer MJ, Balu R et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4):391–404
Dalmau J, Gleichman AJ, Hughes EG et al (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7(12):1091–1098
Mattozzi S, Sabater L, Escudero D et al (2020) Hashimoto encephalopathy in the 21st century. Neurology 94(2):e217–e224
Schiess N, Pardo CA (2008) Hashimoto’s encephalopathy. Ann N Y Acad Sci 1142:254–265
Mirabelli-Badenier M, Biancheri R, Morana G et al (2014) Anti-NMDAR encephalitis misdiagnosed as Hashimoto’s encephalopathy. Eur J Paediatr Neurol 18(1):72–74
Broadley J, Seneviratne U, Beech P et al (2019) Prognosticating autoimmune encephalitis: A systematic review. J Autoimmun 96:24–34
Wang JJ, Li FL, Xiao LL et al (2018) Depressed TSH level as a predictor of poststroke fatigue in patients with acute ischemic stroke. Neurology 91(21):E1971–E1978
Olivecrona Z, Dahlqvist P, Koskinen LO (2013) Acute neuro-endocrine profile and prediction of outcome after severe brain injury. Scand J Trauma Resusc Emerg Med 21:33
Feng GB, Tian X, Wang L et al (2018) Low TT4 as a predictor of poor outcomes in severe encephalitis: a multivariate analysis of 94 patients. Expert Rev Neurother 18(5):443–451
Antonelli A, Ferrari SM, Corrado A et al (2015) Autoimmune thyroid disorders. Autoimmun Rev 14(2):174–180
Lazurova I, Benhatchi K (2012) Autoimmune thyroid diseases and nonorganspecific autoimmunity. Pol Arch Med Wewn 122(Suppl 1):55–59
Chen YL, Yeh JH, Chiu HC (2013) Clinical features of myasthenia gravis patients with autoimmune thyroid disease in Taiwan. Acta Neurol Scand 127(3):170–174
Wang X, Yi H, Liu J et al (2016) Anti-thyroid antibodies and thyroid function in neuromyelitis optica spectrum disorders. J Neurol Sci 366:3–7
Sahraian MA, Owji M, Naser Moghadasi A (2016) Concomitant multiple sclerosis and another autoimmune disease: Does the clinical course change? Clin Neurol Neurosurg 150:92–95
Tuzun E, Erdag E, Durmus H et al (2011) Autoantibodies to neuronal surface antigens in thyroid antibody-positive and -negative limbic encephalitis. Neurol India 59(1):47–50
Lin Y, Tan S, Wang Y et al (2018) Anti-thyroid antibodies and thyroid function in anti-N-methyl-d-aspartate receptor encephalitis. Neurochem Int 113:107–111
Li A, Gong X, Guo K et al (2020) Direct economic burden of patients with autoimmune encephalitis in western China. Neurol Neuroimmunol Neuroinflamm 7(6):e891
van Swieten JC, Koudstaal PJ, Visser MC et al (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5):604–607
Hu F, Yan Z, Ma B et al (2020) The impact of concurrent Hashimoto thyroiditis on thyroid nodule cytopathology assessed by ultrasound-guided fine-needle aspiration cytology. Postgrad Med 132(6):506–511
Lin J, Li C, Li A et al (2019) Encephalitis With Antibodies Against the GABAB Receptor: High Mortality and Risk Factors. Front Neurol 10:1030
Teng W, Shan Z, Teng X et al (2006) Effect of iodine intake on thyroid diseases in China. N Engl J Med 354(26):2783–2793
Caturegli P, De Remigis A, Rose NR (2014) Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev 13(4–5):391–397
Menconi F, Marcocci C, Marino M (2014) Diagnosis and classification of Graves’ disease. Autoimmun Rev 13(4–5):398–402
Titulaer MJ, McCracken L, Gabilondo I et al (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12(2):157–165
Lui DTW, Lee CH, Chow WS et al (2020) Thyroid Dysfunction in Relation to Immune Profile, Disease Status and Outcome in 191 Patients with COVID-19. J Clin Endocrinol Metab 106(2):e926-e935
Suda S, Muraga K, Kanamaru T et al (2016) Low free triiodothyronine predicts poor functional outcome after acute ischemic stroke. J Neurol Sci 368:89–93
Binks S, Varley J, Lee W et al (2018) Distinct HLA associations of LGI1 and CASPR2-antibody diseases. Brain 141(8):2263–2271
Zhao J, Wang C, Xu X et al (2019) Coexistence of Autoimmune Encephalitis and Other Systemic Autoimmune Diseases. Front Neurol 10:1142
Steiner J, Schiltz K, Stoecker W et al (2020) Association of thyroid peroxidase antibodies with anti-neuronal surface antibodies in health, depression and schizophrenia - Complementary linkage with somatic symptoms of major depression. Brain Behav Immun 90:47–54
Mueller SH, Farber A, Pruss H et al (2018) Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann Neurol 83(4):863–869
Zeitlin AA, Heward JM, Newby PR et al (2008) Analysis of HLA class II genes in Hashimoto’s thyroiditis reveals differences compared to Graves’ disease. Genes Immun 9(4):358–363
Bien CG, Vincent A, Barnett MH et al (2012) Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 135(Pt 5):1622–1638
Ajjan RA, Weetman AP (2015) The Pathogenesis of Hashimoto’s Thyroiditis: Further Developments in our Understanding. Horm Metab Res 47(10):702–710
Acknowledgements
The authors thank all participants for their participation in our study.
Funding
Supported by the National Natural Science Foundation of China (grants 82071459).
Author information
Authors and Affiliations
Contributions
J. Lin and J. Wang carried out the statistical analysis and drafted the manuscript. J. Wang collected and interpreted the data. J. Li conceptualized and designed the study and revised the manuscript.
Corresponding author
Ethics declarations
Informed consent
We obtained informed written consent from all participants in this study and publication of their clinical data.
Ethics approval
This study was approved by the Research Ethics Committee of West China Hospital of Sichuan University, and written informed consent was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, JF., Wang, JR., Wang, JQ. et al. The detection of up-regulated anti-thyroid antibodies and autoimmune thyroid diseases in patients with autoimmune encephalitis: a retrospective study of 221 patients. Neurol Sci 43, 3901–3910 (2022). https://doi.org/10.1007/s10072-022-05932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-05932-0