Abstract
Study objectives
This study aims to investigate the extent to which sleep duration and efficiency are associated with plasma amyloid-β (Aβ) levels in non-demented older people.
Methods
This study is a cross-sectional analysis of 305 non-demented older people. Sleep duration and efficiency were assessed used the Pittsburgh Sleep Quality Index. Levels of plasma Aβ were determined by sandwich enzyme-linked immunosorbent assay technique. Associations between sleep variables and plasma Aβ levels were evaluated with multivariable linear regression analysis.
Results
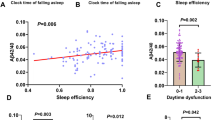
Compared to those with sleep duration > 7 h, participants with sleep duration < 6 h had a higher plasma Aβ42 level (β = 0.495, 95% CI 0.077~0.913, p = 0.021) and Aβ42/Aβ40 ratio (β = 0.101, 95% CI 0.058~0.144, p < 0.001). Compared to those with sleep efficiency ≥ 85%, participants with lower sleep efficiency (65~74%, <65%) had a higher level of plasma Aβ42 (<65%: β = 0.627, 95% CI 0.147~1.108, p = 0.011) and Aβ42/Aβ40 ratio (65~74%: β = 0.052, 95% CI 0.007~0.097, p = 0.026; <65%: β = 0.117, 95% CI 0.067~0.168, p < 0.001).
Conclusions
These findings indicated that short sleep duration and low sleep efficiency were associated with a high level of Aβ42. A better comprehending of the link between sleep and plasma Aβ levels may lead to effective sleep-based intervention to reduce the risk of Alzheimer’s disease.
Similar content being viewed by others
Change history
06 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10072-021-05794-y
References
Wang J, Gu BJ, Masters CL, Wang YJ (2017) A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol 13:703
Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C (1992) Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J Biol Chem 267:546–554
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y (1994) Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). NEURON 13:45–53
Osorio RS, Pirraglia E, Aguera-Ortiz LF et al (2011) Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc 59:559–562
Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA (2013) Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. SLEEP 36:1027–1032
Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM (2013) Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol 70:1537–1543
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. SCIENCE 342:373–377
Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, Bateman RJ (2012) Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol 69:51–58
Slats D, Claassen JA, Verbeek MM, Overeem S (2013) Reciprocal interactions between sleep, circadian rhythms and Alzheimer’s disease: focus on the role of hypocretin and melatonin. Ageing Res Rev 12:188–200
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. SCIENCE 326:1005–1007
Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA (2014) Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol 71:971–977
Lucey BP, Hicks TJ, McLeland JS et al (2018) Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Ann Neurol 83:197–204
Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V, Zehra A, Freeman C, Miller G, Manza P, Srivastava T, de Santi S, Tomasi D, Benveniste H, Volkow ND (2018) beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 115:4483–4488
Huang Y, Potter R, Sigurdson W, Kasten T, Connors R, Morris JC, Benzinger T, Mintun M, Ashwood T, Ferm M, Budd SL, Bateman RJ (2012) beta-Amyloid dynamics in human plasma. Arch Neurol 69:1591–1597
Grimmer T, Laub T, Hapfelmeier A, Eisele T, Fatke B, Hölzle P, Lüscher S, Parchmann AM, Rentrop M, Schwerthöffer D, Müller-Sarnowski F, Ortner M, Goldhardt O, Kurz A, Förstl H, Alexopoulos P (2020) The overnight reduction of amyloid beta 1-42 plasma levels is diminished by the extent of sleep fragmentation, sAPP-beta, and APOE epsilon4 in psychiatrists on call. Alzheimers Dement 16:759–769
Wei M, Zhao B, Huo K, Deng Y, Shang S, Liu J, Li Y, Ma L, Jiang Y, Dang L, Chen C, Wei S, Zhang J, Yang H, Gao F, Qu Q (2017) Sleep deprivation induced plasma amyloid-beta transport disturbance in healthy young adults. J Alzheimers Dis 57:899–906
Sanchez-Espinosa MP, Atienza M, Cantero JL (2014) Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-beta and cortical thinning. Neuroimage 98:395–404
Ju YE, McLeland JS, Toedebusch CD et al (2013) Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70:587–593
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. NEUROLOGY 43:2412–2414
Li H, Jia J, Yang Z (2016) Mini-mental state examination in elderly Chinese: a population-based normative study. J Alzheimers Dis 53:487–496
Pesini P, Perez-Grijalba V, Monleon I et al (2012) Reliable measurements of the beta-amyloid pool in blood could help in the early diagnosis of AD. Int J Alzheimers Dis 2012:604141
Buysse DJ, Reynolds CR, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT III, Derdeyn CP, Bateman RJ (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol 76:837–844
Hanon O, Vidal JS, Lehmann S, Bombois S, Allinquant B, Tréluyer JM, Gelé P, Delmaire C, Blanc F, Mangin JF, Buée L, Touchon J, Hugon J, Vellas B, Galbrun E, Benetos A, Berrut G, Paillaud E, Wallon D, Castelnovo G, Volpe-Gillot L, Paccalin M, Robert PH, Godefroy O, Dantoine T, Camus V, Belmin J, Vandel P, Novella JL, Duron E, Rigaud AS, Schraen-Maschke S, Gabelle A, BALTAZAR study group (2018) Plasma amyloid levels within the Alzheimer’s process and correlations with central biomarkers. Alzheimers Dement 14:858–868
Tzen KY, Yang SY, Chen TF, Cheng TW, Horng HE, Wen HP, Huang YY, Shiue CY, Chiu MJ (2014) Plasma Abeta but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem Neurosci 5:830–836
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K (2018) High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. NATURE 554:249–254
Jagust WJ, Mormino EC (2011) Lifespan brain activity, beta-amyloid, and Alzheimer’s disease. Trends Cogn Sci 15:520–526
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. NEURON 48:913–922
Hartmann T, Bieger SC, Bruhl B et al (1997) Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nat Med 3:1016–1020
Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T (2001) Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis 3:23–30
Domert J, Rao SB, Agholme L, Brorsson AC, Marcusson J, Hallbeck M, Nath S (2014) Spreading of amyloid-beta peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol Dis 65:82–92
Lockley SW, Skene DJ, Arendt J (1999) Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res 8:175–183
Bubu OM, Andrade AG, Umasabor-Bubu OQ, HoganMM Turner AD, de LeonMJ Ogedegbe G, Ayappa I, Jean-Louis GG, Jackson ML, Varga AW, Osorio RS (2020) Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev 50:101250
Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, Hogan MM, Shim AM, Mukhtar F, Sharma N, Mbah AK, Seixas AA, Kam K, Zizi F, Borenstein AR, Mortimer JA, Kip KE, Morgan D, Rosenzweig I, Ayappa I, Rapoport DM, Jean-Louis G, Varga AW, Osorio RS, Alzheimer’s Disease Neuroimaging Initiative (2019) Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep 42:zsz048
Acknowledgements
We would like to thank Ms. Weiping Zhang and Ms. Biqing Lin for the screening tests in our clinic population. We thank Ms. Qiulan Huang for assistance in data collection and recruitment.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC2001700), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2015BAI06B02), and the Special fund of Foshan Summit plan (Grant No. 2019A011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was approved by First People’s Hospital of Foshan Research Ethics Board. The participants provided their written informed consent to participate in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yajing Liu and Lushi Chen are co-first authors.
The original online version of this article was revised: Originally, a number 7 was added in the article title of the article. We have corrected and removed the added data here.
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, L., Huang, S. et al. Sleep duration and efficiency are associated with plasma amyloid-β in non-demented older people. Neurol Sci 43, 305–311 (2022). https://doi.org/10.1007/s10072-021-05271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05271-6