Abstract
Background
Abnormal amyloid β (Aβ) deposits in the brain are a hallmark of Alzheimer’s disease (AD). Insufficient sleep duration and poor sleep quality are risk factors for developing AD. Sleep may play a role in Aβ regulation, but the magnitude of the relationship between sleep and Aβ deposition remains unclear. This systematic review examines the relationship between sleep (i.e., duration and efficiency) with Aβ deposition in later-life adults.
Methods
A search of PubMed, CINAHL, Embase, and PsycINFO generated 5,005 published articles. Fifteen studies met the inclusion criteria for qualitative syntheses; thirteen studies for quantitative syntheses related to sleep duration and Aβ; and nine studies for quantitative syntheses related to sleep efficiency and Aβ.
Results
Mean ages of the samples ranged from 63 to 76 years. Studies measured Aβ using cerebrospinal fluid, serum, and positron emission tomography scans with two tracers: Carbone 11-labeled Pittsburgh compound B or fluorine 18-labeled. Sleep duration was measured subjectively using interviews or questionnaires, or objectively using polysomnography or actigraphy. Study analyses accounted for demographic and lifestyle factors. Based on 13 eligible articles, our synthesis demonstrated that the average association between sleep duration and Aβ was not statistically significant (Fisher’s Z = -0.055, 95% CI = -0.117 ~ 0.008). We found that longer self-report sleep duration is associated with lower Aβ (Fisher’s Z = -0.062, 95% CI = -0.119 ~ -0.005), whereas the objectively measured sleep duration was not associated with Aβ (Fisher’s Z = 0.002, 95% CI = -0.108 ~ 0.113). Based on 9 eligible articles for sleep efficiency, our synthesis also demonstrated that the average association between sleep efficiency and Aβ was not statistically significant (Fisher’s Z = 0.048, 95% CI = -0.066 ~ 0.161).
Conclusion
The findings from this review suggest that shorter self-reported sleep duration is associated with higher Aβ levels. Given the heterogeneous nature of the sleep measures and outcomes, it is still difficult to determine the exact relationship between sleep and Aβ. Future studies with larger sample sizes should focus on comprehensive sleep characteristics and use longitudinal designs to better understand the relationship between sleep and AD.
Similar content being viewed by others
Background
Alzheimer’s disease (AD) is a progressive neurodegenerative disease affecting one in ten adults over the age of 65 worldwide, which poses a considerable economic challenge [1]. More than 6.5 million older Americans suffered from AD in 2022, and the estimated cost for AD is $321 billion worldwide [2]. By 2050, the number of AD cases in the US is expected to reach 12.7 million individuals. Neurodegenerative processes associated with AD result in the accumulation of senile plaques and pathologic changes in Amyloid β (Aβ) throughout the brain, cerebrospinal fluid (CSF), and serum [3, 4]. AD biomarkers may be present even decades before clinical AD symptoms appear [5]. However, few effective disease-modifying treatments exist to delay the onset of AD symptoms. Thus, there is a pressing need to identify modifiable risk factors and develop novel interventions to decrease the risk of AD.
Alterations in sleep duration and efficiency can lead to numerous consequences for health and well-being and increase the risk of AD [6]. Current guidelines state that healthy sleep is a sleep duration of 7 or more hours per night for adults between 18 and 60, 7–9 h for adults between 61 and 64, and 7- 8 h for 65 years and older [7,8,9]. However, later-life adults who are older than 50 typically experience less than 7 h of sleep duration and 85% sleep efficiency compared to younger adults [10]. In particular, slow-wave sleep declines significantly with age [10]. In addition to the changes in sleep structures, sleep disorders including insomnia and sleep-disordered breathing increase with age [10]. Additionally, individuals with mild cognitive impairment or AD often experience disruptions in sleep and experience sundown syndrome. However, this condition often occurs years prior to impairment [11,12,13].
Sleep maintains brain and neural homeostasis [14]. During sleep, the brain controls Aβ peptide regulation [15], clears neurotoxins including Aβ plaques [16], and decreases systematic inflammation [17]. Thus, reductions in sleep duration or disruptions during sleep can influence the pathological changes of Aβ. Numerous recent papers and reviews focusing on the overall direction of sleep have suggested that sleep fragmentation or disruption is associated with AD via Aβ or tau pathology [15, 18,19,20,21]. These findings suggest that improving sleep efficiency and optimal sleep quantity could be an opportunity to prevent and delay AD pathology by decreasing Aβ deposition and tau hyperphosphorylation. Insomnia, sleep disordered breathing, and sleep fragmentation have been found to be associated with the risk of developing AD and related dementia [6]. However, researchers do not fully understand what the optimal sleep duration is to prevent AD.
Prior studies have speculated that shorter sleep duration can be associated with Aβ levels, because both total and partial sleep deprivation have been shown to increase Aβ levels in plasma [22, 23], CSF [24, 25], or brain [26,27,28]. For instance, Zhao et al. (2019) found that chronic sleep restriction was associated with increases in Aβ in a mouse model [28]. Kang et al. (2009) suggested that acute sleep deprivation can increase Aβ levels in animals via orexin regulation [29]. To better understand the magnitude of the relationship between sleep metrics and Aβ, a meta-analysis and/or systematic review is needed. However, few meta-analyses and systematic reviews have specifically focused on how sleep duration and/ or efficiency matters for Aβ accumulation in human studies on adults in later life. The purpose of this systematic review is to focus on the current state of science on how sleep duration/efficiency is associated with Aβ in the brain, CSF, and serum in older adults.
Methods
The purpose of this study is to conduct a systematic review to evaluate how sleep duration and efficiency are associated with Aβ. The study was registered a priori with the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42021266789).
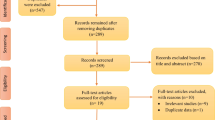
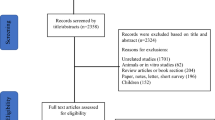
This review was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses [30]. Search strategies were developed with the assistance of a health sciences librarian with expertise in searching for systematic reviews. The flow diagram in Fig. 1 provides details on the search strategy and the number of articles each database yielded. Comprehensive strategies, including both index and keyword methods, were devised by the librarian and the primary author for the following databases: PubMed, CINAHL (EBSCO platform), Embase (Elsevier platform), and PsycINFO (EBSCO platform). To maximize sensitivity, no pre-established database filters other than the English language filter were used. The full PubMed search strategy, as detailed in Supplemental Table A, was also adapted for the other databases. In addition to the database searches, references and cited papers of the 1,156 relevant papers were located using the Scopus database.
Inclusion criteria for the qualitative synthesis were as follows: 1) observational studies with a longitudinal or cross-sectional design, 2) includes exposure variables of sleep duration and/ or sleep efficiency using subjective or objective measures, 3) has Amyloid β plaques (e.g., Aβ, Aβ42, Aβ40, Aβ42/Aβ40) as the outcome, 4) a human study of adults aged 3 50 years old, and 5) recruited (or included) cognitively healthy individuals. An additional inclusion criterion for the quantitative synthesis was studies that reported sufficient data for examining the effect sizes, such as Pearson’s correlation (r), regression coefficient (β), means, standard deviations, t, F, or X2 values. We excluded studies 1) not written in English, 2) interventional studies, 3) non-peer reviewed papers, proceedings, editorials, and reviews, and 4) the study sample focused only on neurological conditions or sleep disorders. For the quantitative synthesis, we excluded studies that lacked or had inadequate inferential statistical results for calculating the effect size.
The initial search yielded 6,987 articles. After removing 1,982 duplicate articles, 5,005 articles were imported to the web-based systematic review application, Rayyan. The level of agreement between authors was determined using Cohen’s κ statistics. Four authors (CM, KV, HD, MZ) screened the abstracts and titles of the 5,005 articles based on the eligibility criteria (k = 0.49). Then, additional articles were removed leaving 62 full-text articles that were reviewed by four authors (CM, AS, YC, MZ) (k = 0.97). A total of 15 articles met the inclusion criteria for the qualitative synthesis and 13 studies met the criteria for quantitative synthesis of sleep duration, and 9 studies for sleep efficiency (Fig. 1 ). Disagreements were resolved through discussion among all authors until consensus was reached.
Quality analysis
The risk bias of the selected papers was assessed independently by two reviewers using the National Institute of Health Study Quality Assessment Tool (2019) for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The internal validity of the studies were assessed based on 14 domains: 1) bias due to an unclear purpose, 2) bias due to an unclear specification of the population, 3) bias due to ineligible participants, 4) bias due to recruitment from a different population, 5) bias due to unclear power justification, 6) bias due to measure timing, 7) bias due to time frame, 8) bias due to outcome level, 9) bias due to invalid exposure measure, 10) bias due to frequency of the assessments, 11) bias due to the outcome measure, 12) bias due to an unblinded assessment, 13) bias due to loss during follow up, and 14) bias due to statistical analysis and confounding. For each domain, we categorized the risk of bias as either low or high risk. We rated an item “unclear risk” if there was no information about the risk of bias.
Statistical analysis
We aggregated the effect sizes across the studies and calculated the publication bias, overall effect sizes, and Q statistics using Comprehensive Meta-Analysis (CMA) version 4 software (Biostat, Inc). We also calculated the effect sizes using Fisher’s Z as the effect size index after examining the available information on the correlation between sleep duration/sleep efficiency and Aβ using Fisher’s Z = 0.5*Log(1 + Corr)/(1-Corr) [31]. We used a Q statistic to evaluate the heterogeneity of variance. We also calculated the I 2 index using I 2 = 100% × (Q − degree of freedom)/Q to identify how the variance in observed effects reflected the variance in true effects rather than by random error. The random-effects model was applied in the current study because we expected that the sampling distribution varied across the studies and parameters were drawn from random variables [32,33,34,35,36]. We also ran subgroup analysis based on the sleep measure (i.e., self-report vs. objective). We used meta-regression analyses to investigate quantitative relationships between the dependent variable and covariate (i.e., sex). To consider the possibility of sampling bias from all possible samples, we assessed the studies for publication bias. First, we visually inspected the studies for symmetry of the funnel plot (Supplemental Figure A, B). Second, we ran Tweedie's Trim and Fill test to ensure that the publication bias could not reverse our estimate of the effect sizes [31].
Results
Fifteen articles were included in the qualitative synthesis portion of this review. Thirteen studies were included for the quantitative synthesis of sleep duration and Aβ levels, and nine studies were included for quantitative synthesis of sleep efficiency and Aβ levels. Table 1 summarizes the study sample, design, and assessments of each study. Table 2 summarizes the study results and relevant information. Table 3 summarizes meta-analysis results.
Qualitative synthesis
Study characteristics
The total number of participants of the 15 studies included in the qualitative synthesis was 11,295 individuals ranging from 13 to 4,712 participants. The overall demographics of this review are presented in Table 1. The countries included the United States (n = 5) [37,38,39,40, 42], Australia [41], France [44], Italy [45], Netherlands [46], South Korea [43], and China [48,49,50]. Winer and colleagues’ study collected data from participants in multiple countries, including the United States, Canada, Australia, and Japan [47]. Blackman et al. also involved 39 European organizations [51]. Most of the studies used a cross-sectional study design [37,38,39,40,41,42,43,44,45,46,47,48,49,50], and one study used a longitudinal design [51].
The mean age of the samples ranged from 61.6 to 75.7. Data from the studies included study subjects, and most studies had specific inclusion criteria for the mean age and cognitive status [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The rate of females included in the sample ranged from 42 to 71%. Except for the studies by Gabelle et al. and Fu et al., more females were included in the studies than male participants. All studies only included individuals who were cognitively healthy without any neurological or untreated psychological conditions or certain health conditions that may affect sleep and Aβ. Exclusion criteria for all studies in our analysis were low cognition or markers associated with cognitive impairment such as lesions, stroke, or neurological disorders [37,38,39,40,41,42,43,44,45, 47,48,49,50,51], other major illnesses [39, 42, 45, 48, 50], and drugs that are active in the CNS [39].
Quality assessment
Figure 2 illustrates the assessment of the risk of bias categories. Among the 15 studies, three had a moderate to high risk of bias due to measurement timing, the outcome measure, exposure measure, or the population and participants. Two of the 15 articles had a risk of bias related to a small sample size and population without any power justification. Two of the studies reported a high risk of bias related to the measurement timing, timeframe, and outcome and exposure variable. Ten of the 15 articles had a risk of bias related to the exposure measure using a self-report sleep question or questionnaire. Five articles had a risk of bias due to the limited number of confounding variables.
Sleep measures
Both subjective and objective sleep measures were used in the reviewed studies (Table 1). Five studies used objective measurements, including polysomnography (PSG) [39, 42] and actigraphy [37, 43, 45]. Overnight data were collected in studies with PSG. Of the three articles assessing sleep duration and sleep efficiency with actigraphy, two of the studies used Actiwatch 2 (Phillips Respironics) [37, 43]. Ettore et al. (2019) used a three-axis accelerometer (GT3X + , Actigraph Corp, Pensacola, FL). All actigraphy data were collected in 60-s epochs, and the duration of the actigraphy recording ranged from 6 to 14 days.
Ten of the reviewed studies used subjective measurements including the Pittsburgh Sleep Quality Index (n = 6) [41, 46, 48,49,50,51], a standardized interview to assess sleep duration (n = 3) [38, 44, 47], and the sleep scale in the Medical Outcomes Study (n = 1) [40]. The interviews included questions related to the duration of nighttime sleep, daytime sleep, total sleep time (daytime and nighttime sleep), and sleep efficiency [44]. Six articles used sleep duration as a categorical variable. Spira et al. coded the categorized sleep variable in a continuous manner by coding 0 for sleep duration longer than 7 h a night, and 3 for sleep duration of 5 h or less. The other two papers by Gabelle et al. and Winer et al. categorized sleep duration as shorter (6 h or less), normal (6—7 h and 6—8 h), and longer (≥ 7 h or ≥ 9 h) sleep duration. Chu et al. categorized sleep duration by the interquartile range (< 5 h, ≥ 5 h to < 6 h, ≥ 6 h to < 7 h, ≥ 7 h to < 8 h, > 8 h), and then used a dichotomous variable of sleep duration less than or equal to 8 and greater than 8 h. Blackman et al. also categorized sleep duration into < 5 h, 5-6 h, 6–7 h, and ≥ 7 h. The time window for the sleep duration assessment varied from 1 day to four weeks. Ten articles treated the sleep duration variable as a continuous variable. Ten of the reviewed studies reported sleep efficiency [37, 41,42,43,44,45,46, 48, 50, 51]. Of those articles, three studies categorized sleep efficiency [44, 48, 51] and the other seven studies treated sleep efficiency as a continuous variable.
Amyloid measures
The reviewed studies used a variety of Aβ measures (Table 1). Six studies measured Aβ concentration from bodily fluids including CSF (n = 4) [37, 42, 49, 51] and peripheral blood (n = 2) [46, 48]. CSF was obtained by lumbar puncture the morning after overnight fasting (from 8 to 10am) [37] or CSF samples collected between 11:00–13:00 [42]. In the two studies that tested peripheral blood, samples were collected in the morning after overnight fasting [46, 48], and were processed using plasma for further analysis. These studies measured Aβ40 and Aβ 42 and assessed different combined ratios (e.g., Aβ 42,/ Aβ40, P-tau/ Aβ 42, T-tau/ Aβ 42, NFL/ Aβ 42)[37, 42, 46, 48, 49] by an enzyme-linked immunosorbent assay (ELISA) [37, 42, 48,49,50] or by Simoa [46].
Nine studies measured Aβ using a PET scan [38,39,40,41, 43,44,45, 47, 50]. These studies obtained the amyloid deposit using two tracers: four studies used Carbone11 labeled Pittsburgh compound B (11C-PiB) [39,40,41, 43], six studies used fluorine 18 (18F) labeled tracers including 18F-florbetapir [38, 41, 44, 45, 47, 50], and one study used 18F-flutemetamol [41]. Brown et al. (2016) utilized data using three tracers: [C-11] PiB, and 18F-florbetapir, and 18F-flutemetamol [41].
Studies that conducted a quantitative assessment of Aβ used standardized uptake value ratios (SUVr) in four studies [41, 43,44,45], and the distribution volume ratio (DVR) in three studies [39, 40, 47]. Three studies with PET brain imaging used a cutoff to determine amyloid positivity. Ettore et al. (2019) used SUVr of 0.7918, Gabelle et al. (2019) used SUVr of 1.17, and Hwang et al. (2018) used SUVr of 1.21. Ju et al. (2013) used CSF Aβ42 of 500 pg/ml for the cut off.
Table 1 presents the covariate adjustments used in the statistical analyses of the studies. In general, the studies accounted for age and sex, except for Gabelle et al. (2019). Race and ethnicity were accounted for in two studies [38, 47]. Eleven studies controlled for APOEε4 allele [37, 38, 40, 42,43,44, 47,48,49,50,51]. Several studies also adjusted for education [41, 42, 46,47,48,49,50]. Clinical factors that were accounted for in the studies included depression [38, 41, 43,44,45, 48], and cognition status measured by the mini-mental state examination (MMSE) [41, 45, 48] or Montreal Cognitive Assessment (MOCA) [50]. Body mass index was a common lifestyle covariate considered in several studies [38,39,40,41, 46, 48, 50]. Other lifestyle covariates included hypertension [46, 48,49,50] and diabetes [46, 48,49,50]. Sleep and circadian rhythm variables were also included such as slow wave sleep [42], sleep disturbance [50], sleep apnea [46], or sleep medication [39]. Other factors included family history of AD, alcohol, and caffeine consumption [46, 49, 50], cholesterol levels [46, 48], and exercise [48].
Association between exposure and outcomes
Table 2 describes the findings of the reviewed studies for the qualitative synthesis. Five of the 15 articles [38, 47,48,49,50] found that shorter sleep duration was associated with higher Aβ. However, four of the studies reported the reverse association between sleep duration and PET-measured global and regional Aβ burden. Winer et al., (2021) found that self-reported shorter sleep duration was associated with greater 18 F-florbetapir-PET brain imaging derived DVR Aβ burden (β = –0.01; p = 0.005). Spira et al. (2014) also reported that shorter sleep duration was associated with greater Aβ burden, measured by mean cortical [C-11] PiB PET derived DVR (cDVR; β = 0.08, p = 0.005) and precuneus DVR (β = 0.11, p = 0.007). Longer total sleep time was associated with reduced 18 F-florbetapir-PET brain imaging derived SUVr global Aβ (β = -0.005; p = 0.03), reduced medial orbitofrontal Aβ (β = -0.009; p < 0.001), and reduced anterior cingulate Aβ (β = -0.011; p < 0.001). Sleep duration longer than 8 h was associated with having higher amyloid burden compared to sleep duration shorter or equal to 8 h (Odds Ratio = 4.167; p = 0.020) [50]. In addition to studies using PET, findings from a Chinese sample by Liu et al. (2021) also found that shorter sleep duration was associated with higher plasma Aβ42 (β = 0.495, p = 0.021) and Aβ42/Aβ40 ratio (β = 0.101, p < 0.001) [48]. Fu et al. (2021) also found a non-linear relationship indicating a decrease in CSF Aβ42 with shorter or longer sleep duration, with the extreme point being 6.23 h of sleep [49]. However, Blackman et al. (2023)’s cross-sectional analysis and longitudinal analysis did not identify any association between sleep duration and Aβ42 although the authors found significant associations between sleep characteristics and CSF P-tau and t-tau [51].
Of the reviewed studies, ten of the studies investigated sleep efficiency [15, 41,42,43,44,45,46, 48, 50, 51]. Three of the studies reported that sleep efficiency was associated with Aβ burden. Ettore et al. (2019) showed that lower sleep efficiency was found in the Aβ positive group, and increased sleep efficiency was associated with a 41% reduction of Aβ positivity (Odds Ratio = 0.59, < 0.001). Ju et al. (2013) reported that individuals with a low CSF Aβ42 level (≤ 500 pg/mL), which is indicative of amyloid deposition in the brain, had worse sleep efficiency than those with a normal CSF Aβ42 level (80.4% vs. 83.7%, p = 0.08), although sleep duration was not statistically significant. Liu et al. (2021) reported that sleep efficiency was negatively associated with the plasma Aβ42 level (β = − 0.025, 95% CI − 0.037 ~ − 0.013, p = 0.001) and Aβ42/Aβ40 ratio (β = − 0.004, 95% CI − 0.005 ~ − 0.002, p < 0.001). Specifically, experiencing less than 65% of sleep efficiency was positively associated (β = 0.125, 95% CI 0.077 ~ 0.173, p < 0.001) with plasma the Aβ42 level compared to sleep efficiency greater or equal to 85%. In particular, sleep efficiency between 65 and 74% was positively associated (β = 0.434, 95% CI 0.025 ~ 0.844, p = 0.038) with plasma Aβ42 level compared to sleep efficiency greater or equal to 85%.
Quantitative synthesis
Thirteen eligible articles were used for the quantitative synthesis because the remaining studies were not included due to a lack of reporting information to calculate Fisher’s Z value. Because one study included both objective and subjective sleep data, we included objective sleep information to calculate the overall associations. However, we included the effect size information based on the self-report measures when we conducted the subgroup analysis. Figure 3 and Table 3 summarize the quantitative synthesis of the articles.
The findings demonstrate that the average association between sleep duration and Aβ was not statistically significant (Fisher’s Z = -0.055, 95% CI = -0.117 ~ 0.008) (Fig. 3A). The Z-value was -1.720 with p = 0.085. As shown in Table 3, for heterogeneity, our results indicate a Q-value of 44.44 with 12 degrees of freedom. The amount of between-study variance in the observed effect was less than we expected based on sampling error alone. The I2 statistic was 73%, indicating that 73% of the variance in observed effects reflect the variance in true effects rather than sampling error. Tau reflects the standard deviation of the true effect size, which is 0.007 in Fisher’s Z units.
For the subgroup analysis (Fig. 3B) based on the sleep measurement, we found that the average association between self-reported sleep duration and Aβ was significant. This finding indicates that longer self-reported sleep duration is associated with lower Aβ (Fisher’s Z = -0.062, 95% CI = -0.119 ~ -0.005) with a Z-value of -2.146 (p-value = 0.032) in nine studies. However, the average association between objectively measured sleep duration and Aβ was not statistically significant (Fisher’s Z = 0.002, 95% CI = -0.108 ~ 0.113) with a Z value of 0.038 (p-value = 0.969) in 5 studies. Furthermore, meta-regressions showed no impact of sex (coefficient = 0. 288; CI = -0.674 ~ 1.249) with Z value of 0.59 (p-value = 0.557). As shown in the standard error funnel plot by Fisher’s Z (Supplemental Figure A), the plot is slightly asymmetric, indicating that there could be minor publication bias from the included studies. This might be due to either our inability to identify studies with non-significant finings or failing to report non-significant findings [31]. Thus, we conducted an analysis using Tweedie’s Trim and Fill method for the overall relationship between sleep duration and Aβ, which demonstrated that even if we removed one study, the effect size remained statistically insignificant (Fisher’s Z = -0.054, 95% CI = -0.117 ~ 0.008). This finding may imply that some of the articles may not have presented the findings of non-significant results.
For sleep efficiency, the findings from nine studies demonstrated that the average association between sleep efficiency and Aβ was not statistically significant (Fisher’s Z = 0.048, 95% CI = -0.066 ~ 0.161) (Fig. 4A). Figure 4 and Table 3 summarize the quantitative synthesis of the articles. The Z-value was 0.823 with p = 0.410. The Q-value is 66.532 with 8 degrees of freedom and p < 0.001. Using a criterion alpha of 0.100, we can reject the null hypothesis that the true effect size is the same in all of these studies. The I2 statistic was 88% and Tau-squared was 0.024 in Fisher's Z units, and Tau was 0.154 in Fisher's Z units. For the subgroup analysis based on the sleep measurement (Fig. 4B), we found that the average association between self-report sleep efficiency and Aβ was not significant (Fisher’s Z = -0.007, 95% CI = -0.126 ~ 0.113) with Z value of -0.107 (p-value = 0.915) in five studies. However, the average association between objectively measured sleep efficiency and Aβ was not statistically significant (Fisher’s Z = 0.085, 95% CI = -0.054 ~ 0.225) with a Z value of 1.199 (p-value = 0.230) in five studies. However, the meta-regression results indicated that higher proportion of female was associated with the higher correlation of sleep efficiency and Aβ (coefficient = 1.746, 95% CI = 0.345 ~ 3.136) with Z-value of 2.44 (p-value = 0.015). As shown in the standard error funnel plot by Fisher’s Z (Supplemental Figure B), the plot is slightly asymmetric, indicating that there could be minor publication bias from the included studies. This bias could be due to either our inability to identify studies with non-significant finings or failing to report non-significant findings [31]. We ran Tweedie’s Trim and Fill method for the overall relationship between sleep duration and Aβ, which demonstrated that even if we remove one study, the effect size remains statistically insignificant (Fisher’s Z = 0.048, 95% CI = -0.066 ~ 0.161). This finding may imply that some of the articles might not have presented the findings due to non-significant results.
Discussion
This review synthesized fifteen studies for the qualitative synthesis and 13 studies for the quantitative synthesis focusing on sleep duration and Aβ and 9 studies for focusing on sleep efficiency and Aβ. This review adds to the current literature with an overview of the measurements and findings related to sleep and Aβ levels. Our meta-analysis findings indicate that there was a significant inverse relationship between self-report sleep duration and Aβ levels, indicating that self-report shorter sleep duration was associated with greater Aβ levels, with Fisher’s Z = -0.062 (95% CI = -0.119 ~ -0.005, p = 0.032). However, we did not find a significant overall relationship between sleep duration and Aβ levels. We also did not find a significant overall relationship between sleep efficiency and Aβ levels. Due to the heterogeneity among the published studies, no firm conclusions could be drawn.
We only found a significant inverse relationship between self-report sleep duration and Aβ levels. Prior research has demonstrated that chronic sleep restriction or deprivation of slow wave sleep can alter the diurnal fluctuation of CSF Aβ levels [24, 25, 52, 53]. Sleep deprivation may also impair human memory consolidation, in part by reducing the synthesis of proteins needed to support synaptic plasticity [14, 54,55,56]. In a meta-analysis by Wu and colleagues (2018), the authors suggested that there is a U-shaped relationship between sleep duration and cognitive disorders. Compared to the reference group (7 – 8 h per day), individuals with short or long duration had a higher risk of developing cognitive disorders, such as Alzheimer’s disease or dementia [57]. Both shorter sleep duration (< 7 h / night) and poor subjective sleep quality are important for cognitive function [58] and brain structures and functions [59, 60]. Furthermore, higher amyloid burden may be associated with worse sleep recollection in the self-report measures. However, more studies are needed using larger sample sizes, and a prospective design. Future studies on the magnitude of correlations of null results can shed light on the true relationship between sleep duration and Aβ.
The overall quantitative synthesis of sleep (duration and sleep efficiency) and Aβ revealed an effect size of -0.055 and 0.048, but they were not statistically significant, indicating that sleep may not be a primary factor in Aβ accumulation. Alternatively, these results may be due to moderators (i.e., APOE4, sex, age, family history, or unmeasured moderators), heterogenous outcome types of Aβ that may not provide consistent ideas, or publication bias due to insignificant results not being favorable for publication. There was considerable heterogeneity in the methods used in the reviewed studies to identify the relationship between sleep (duration and efficiency) and Aβ burden. It is intriguing that the results from the subgroup analysis differed when studying sleep duration and Aβ in studies using subjective and objective sleep measures. Future studies are needed to capture habitual sleep duration using an actigraphy with verification using a sleep diary for 7 to 14 days [61]. The use of a polysomnography would also provide insights on the structure of sleep and sleep disorders [62,63,64].
The majority of the studies used sleep duration as a continuous variable using multi-variate linear regression models. Winer et al. (2021), and Liu et al. (2021) used a categorial variable in the model and provided insights on the dose-dependent relationship between sleep duration and Aβ [47, 48]. Both studies indicated that a shorter sleep duration compared to the standard 7–8 h a night or 7 h or more sleep is associated with greater Aβ. Among the reviewed studies, only Fu and colleagues reported a non-linear relationship between sleep duration and CSF measured Aβ42 demonstrating lower Aβ42 values for shorter or longer sleep and the highest for 6.23 h [49]. Future studies using comprehensive and accurate assessments of sleep as well as a non-linear model would provide deeper insights on recommendations for sleep duration.
In addition to the considerable variability in sleep measurements, Aβ was also measured in different ways: CSF, serum sample, and PET to quantify the Aβ burden. Studies using PET used different tracers (e.g., 11C-PiB, 18F-florbetapir, 18F-flutemetamol) as well as different quantification methods. Most studies focused on global Aβ burden in the brain, but assessing both the overall levels of Aβ in PET as well as specific regional deposition could help us understand areas of the brain that may be affected more than other areas. This variability across the measurements prevented us drawing strong conclusions. However, it is promising in the current field of science to review data across different measures of Aβ accumulation. Although AD can be diagnosed at an autopsy [65], the US National Institute on Aging and Alzheimer’s Association has suggested using Aβ as well as tau and neurodegeneration to define and diagnose AD in both symptomatic and asymptomatic stages [66]. Increased accessibility to biomarkers and the potential for blood biomarkers or additional biomarkers in addition to Aβ would provide further information about the underlying disease progression in the future.
In addition to duration and efficiency of sleep, other sleep dimensions could be important factors for Aβ accumulation. The reviewed studies also identified a positive link between Aβ and different sleep characteristics including less adequate sleep, more sleep problems, and greater somnolence based on participants’ self-report perceptions [40], sleep quality [38], frequent napping [37], longer sleep latency [41, 45], greater sleep fragmentation [45], a higher apnea hypopnea index, and slow wave sleep time [39, 42]. These results may indicate that different dimensions of sleep could contribute more to Aβ burden than quantity of sleep.
The reviewed studies accounted for various demographic and clinical confounders in the multivariate models. Interestingly, we found that sex distribution could impact the relationship between sleep efficiency and Aβ although sex was not a significant moderator on the relationship between sleep duration and Aβ. Most of the reviewed studies accounted for age and sex, which are well-known confounders [10, 67,68,69]. Further stratified analysis or moderation analysis based on sex and age group will provide more accurate understanding of the covariates. Individuals with sleep disorders, underlying health or psychological conditions, medications, genetic factors, social determinants, high fat diets, and physical activity have different sleep quantity and quality [70,71,72]. These factors may also increase the amount of Aβ accumulation [73,74,75,76,77,78,79,80]. These confounding factors may play critical roles in determining the association between sleep duration and Aβ burden. However, these studies used a cross-sectional design, which prevents us from determining causal relationships. Specifically, some of the studies did not measure sleep and Aβ burden in a similar time period, so the results may not reflect a direct link between the two factors. Although researchers have speculated that the relationship between sleep and AD pathology could be bidirectional, there is limited evidence to support the longitudinal relationships [21, 81].
The strength of this review is that we examined current evidence related to sleep and Aβ. However, there are a few limitations. First, the study did not test for moderating effects of age, sex, or APOE4 status. Second, the current study only included publications written in English even though some important findings may have been published in different languages. Third, this review focused on sleep duration and efficiency, but other specific sleep characteristics could have more influence on Aβ pathology.
Conclusions
The results of this systematic review suggest an inverse association between self-report sleep duration and Aβ. However, the relationships between sleep duration and Aβ accumulation as well as sleep efficiency and Aβ accumulation should be interpreted with caution. Researchers would greatly benefit from more studies using a longitudinal design, comprehensive sleep measure, a broad range of biomarkers, and larger sample sizes to advance scholarly understanding of the relationship between sleep and AD.
Availability of data and materials
Data are available upon request to the corresponding author.
Abbreviations
- Aβ:
-
Amyloid β
- AD:
-
Alzheimer’s disease
- CSF:
-
Cerebrospinal fluid
- CMA:
-
Comprehensive Meta-Analysis
- DVR:
-
Distribution volume ratio
- MMSE:
-
The mini-mental state examination
- MOCA:
-
Montreal Cognitive Assessment
- PET:
-
Positron emission tomography
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSG:
-
Polysomnography
- SUVr:
-
Standardized uptake value ratios
- 11C-PiB:
-
Carbone11 labeled Pittsburgh compound B
- 18F:
-
Fluorine 18
References
Patterson C. World Alzheimer Report 2018: The state of the art of dementia research: New frontiers. 2018.
Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement J Alzheimers Assoc. 2021;17:1966–75. https://doi.org/10.1002/alz.12362.
Folch J, Ettcheto M, Petrov D, Abad S, Pedrós I, Marin M, et al. Review of the advances in treatment for Alzheimer disease: strategies for combating β-amyloid protein. Neurol Engl Ed. 2018;33:47–58. https://doi.org/10.1016/j.nrleng.2015.03.019.
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:280–92. https://doi.org/10.1016/j.jalz.2011.03.003.
Shi L, Chen S-J, Ma M-Y, Bao Y-P, Han Y, Wang Y-M, et al. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. https://doi.org/10.1016/j.smrv.2017.06.010.
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–3. https://doi.org/10.1016/j.sleh.2014.12.010.
Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2016;12:785–6. https://doi.org/10.5664/jcsm.5866.
Consensus Conference Panel, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2015;(11):591–2. https://doi.org/10.5664/jcsm.4758.
Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73.
Klaffke S, Staedt J. Sundowning and circadian rhythm disorders in dementia. Acta Neurol Belg. 2006;106:168–75.
McCurry SM, Ancoli-Israel S. Sleep Dysfunction in Alzheimer’s Disease and Other Dementias. Curr Treat Options Neurol. 2003;5:261–72. https://doi.org/10.1007/s11940-003-0017-9.
Yesavage JA, Noda A, Heath A, McNerney MW, Domingue BW, Hernandez Y, et al. Sleep-wake disorders in Alzheimer’s disease: further genetic analyses in relation to objective sleep measures. Int Psychogeriatr. 2019:1–7. https://doi.org/10.1017/S1041610219001777.
Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. https://doi.org/10.1016/j.neuron.2013.12.025.
Ju Y-ES, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology - a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. https://doi.org/10.1126/science.1241224.
Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019;18:296–306. https://doi.org/10.1016/S1474-4422(18)30450-2.
Mander BA. Local Sleep and Alzheimer’s Disease Pathophysiology. Front Neurosci. 2020;14:525970.
Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016;39:552–66. https://doi.org/10.1016/j.tins.2016.05.002.
Proserpio P, Arnaldi D, Nobili F, Nobili L. Integrating Sleep and Alzheimer’s Disease Pathophysiology: Hints for Sleep Disorders Management. J Alzheimers Dis JAD. 2018;63:871–86. https://doi.org/10.3233/JAD-180041.
Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2020;45:104–20. https://doi.org/10.1038/s41386-019-0478-5.
Wei M, Zhao B, Huo K, Deng Y, Shang S, Liu J, et al. Sleep Deprivation Induced Plasma Amyloid-β Transport Disturbance in Healthy Young Adults. J Alzheimers Dis JAD. 2017;57:899–906. https://doi.org/10.3233/JAD-161213.
Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Sleep deprivation and plasma biomarkers for Alzheimer’s disease. Sleep Med. 2019;57:92–3. https://doi.org/10.1016/j.sleep.2018.12.029.
Blattner MS, Panigrahi SK, Toedebusch CD, Hicks TJ, McLeland JS, Banks IR, et al. Increased cerebrospinal fluid amyloid-β during sleep deprivation in healthy middle-aged adults is not due to stress or circadian disruption. J Alzheimers Dis JAD. 2020;75:471–82. https://doi.org/10.3233/JAD-191122.
Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JAHR. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–7. https://doi.org/10.1001/jamaneurol.2014.1173.
Brice KN, Hagen CW, Peterman JL, Figg JW, Braden PN, Chumley MJ, et al. Chronic sleep restriction increases soluble hippocampal Aβ-42 and impairs cognitive performance. Physiol Behav. 2020;226:113128. https://doi.org/10.1016/j.physbeh.2020.113128.
Kincheski GC, Valentim IS, Clarke JR, Cozachenco D, Castelo-Branco MTL, Ramos-Lobo AM, et al. Chronic sleep restriction promotes brain inflammation and synapse loss, and potentiates memory impairment induced by amyloid-β oligomers in mice. Brain Behav Immun. 2017;64:140–51. https://doi.org/10.1016/j.bbi.2017.04.007.
Zhao B, Liu P, Wei M, Li Y, Liu J, Ma L, et al. Chronic Sleep Restriction Induces Aβ Accumulation by Disrupting the Balance of Aβ Production and Clearance in Rats. Neurochem Res. 2019;44:859–73. https://doi.org/10.1007/s11064-019-02719-2.
Kang J-E, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. https://doi.org/10.1126/science.1180962.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9W64;151:264-9W 64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 2nd ed. Hoboken: Wiley; 2021.
Borenstein M. Common Mistakes in Meta-Analysis and How to Avoid Them. Englewood, NJ: Biostat Inc.; 2019.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. https://doi.org/10.1002/jrsm.12.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane. 2021. Available from: training.cochrane.org/handbook.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. https://doi.org/10.1002/sim.1186.
Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. https://doi.org/10.1037/1082-989X.3.4.486.
Ju Y-ES, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. https://doi.org/10.1001/jamaneurol.2013.2334.
Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-Reported Sleep and β-Amyloid Deposition in Community-Dwelling Older Adults. JAMA Neurol. 2013;70:1537–43. https://doi.org/10.1001/jamaneurol.2013.4258.
Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, et al. Objectively Measured Sleep and β-amyloid Burden in Older Adults: A Pilot Study. SAGE Open Med. 2014;2. https://doi.org/10.1177/2050312114546520.
Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568–76. https://doi.org/10.1016/j.neurobiolaging.2015.05.004.
Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, et al. The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep. 2016;39:1063–8. https://doi.org/10.5665/sleep.5756.
Varga AW, Wohlleber ME, Giménez S, Romero S, Alonso JF, Ducca EL, et al. Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Aβ42 Levels in Cognitively Normal Elderly. Sleep. 2016.
Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi J-W, et al. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology. 2018;90:e1167–73. https://doi.org/10.1212/WNL.0000000000005193.
Gabelle A, Gutierrez L-A, Jaussent I, Ben Bouallegue F, De Verbizier D, Navucet S, et al. Absence of Relationship Between Self-Reported Sleep Measures and Amyloid Load in Elderly Subjects. Front Neurol. 2019;10:989. https://doi.org/10.3389/fneur.2019.00989.
Ettore E, Bakardjian H, Solé M, Levy Nogueira M, Habert M-O, Gabelle A, et al. Relationships between objectives sleep parameters and brain amyloid load in subjects at risk to Alzheimer’s disease: the INSIGHT-preAD Study. Sleep. 2019. https://doi.org/10.1093/sleep/zsz137.
Lysen TS, Ikram MA, Ghanbari M, Luik AI. Sleep, 24-h activity rhythms, and plasma markers of neurodegenerative disease. Sci Rep. 2020;10:20691. https://doi.org/10.1038/s41598-020-77830-4.
Winer JR, Deters KD, Kennedy G, Jin M, Goldstein-Piekarski A, Poston KL, et al. Association of Short and Long Sleep Duration With Amyloid-β Burden and Cognition in Aging. JAMA Neurol. 2021;78:1187–96. https://doi.org/10.1001/jamaneurol.2021.2876.
Liu Y, Chen L, Huang S, Lv Z, Hu L, Luo J, et al. Sleep duration and efficiency are associated with plasma amyloid-β in non-demented older people. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;43:305–11. https://doi.org/10.1007/s10072-021-05271-6.
Fu Y, Wang Z-T, Qu Y, Wang X-T, Ma Y-H, Bi Y-L, et al. Sleep Characteristics and Cognitive Function in Older Adults Without Dementia: The CABLE Study. J Alzheimers Dis JAD. 2022;84:1029–38. https://doi.org/10.3233/JAD-215017.
Chu H, Huang C, Miao Y, Ren C, Guan Y, Xie F, et al. The association of subjective sleep characteristics and plasma biomarkers of Alzheimer’s disease pathology in older cognitively unimpaired adults with higher amyloid-β burden. J Neurol. 2023. https://doi.org/10.1007/s00415-023-11626-0.
Blackman J, Stankeviciute L, Arenaza-Urquijo EM, Suárez-Calvet M, Sánchez-Benavides G, Vilor-Tejedor N, et al. Cross-sectional and longitudinal association of sleep and Alzheimer biomarkers in cognitively unimpaired adults. Brain Commun. 2023;4:fcac257. https://doi.org/10.1093/braincomms/fcac257.
Ju Y-ES, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain J Neurol. 2017;140:2104–11. https://doi.org/10.1093/brain/awx148.
Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, et al. Effect of sleep on overnight CSF amyloid-β kinetics. Ann Neurol. 2018;83:197–204. https://doi.org/10.1002/ana.25117.
Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci Off J Soc Neurosci. 2013;33:14288–300. https://doi.org/10.1523/JNEUROSCI.5102-12.2013.
Durmer JS, Dinges DF. Neurocognitive Consequences of Sleep Deprivation. Semin Neurol. 2005;25:117–29. https://doi.org/10.1055/s-2005-867080.
Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–43. https://doi.org/10.1016/j.sleep.2007.03.011.
Wu L, Sun D, Tan Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath. 2018;22:805–14. https://doi.org/10.1007/s11325-017-1527-0.
Lucey BP, Wisch J, Boerwinkle AH, Landsness EC, Toedebusch CD, McLeland JS, et al. Sleep and longitudinal cognitive performance in preclinical and early symptomatic Alzheimer’s disease. Brain J Neurol. 2021;144:2852–62. https://doi.org/10.1093/brain/awab272.
Baril A-A, Beiser AS, Mysliwiec V, Sanchez E, DeCarli CS, Redline S, et al. Slow-Wave Sleep and MRI Markers of Brain Aging in a Community-Based Sample. Neurology. 2021;96:e1462–9. https://doi.org/10.1212/WNL.0000000000011377.
De Looze C, Feeney JC, Scarlett S, Hirst R, Knight SP, Carey D, et al. Sleep duration, sleep problems, and perceived stress are associated with hippocampal subfield volumes in later life: findings from The Irish Longitudinal Study on Ageing. Sleep. 2022;45:zsab241. https://doi.org/10.1093/sleep/zsab241.
Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, et al. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav Sleep Med. 2015;13(Suppl 1):S4-38. https://doi.org/10.1080/15402002.2015.1046356.
Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379–88.
Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. n.d.;14:1231–7. https://doi.org/10.5664/jcsm.7230.
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. n.d.;13:479–504. https://doi.org/10.5664/jcsm.6506.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. https://doi.org/10.1212/wnl.34.7.939.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Dorsey A, de Lecea L, Jennings KJ. Neurobiological and Hormonal Mechanisms Regulating Women’s Sleep. Front Neurosci. 2020;14:625397. https://doi.org/10.3389/fnins.2020.625397.
Guo L, Zhong MB, Zhang L, Zhang B, Cai D. Sex Differences in Alzheimer’s Disease: Insights From the Multiomics Landscape. Biol Psychiatry. 2022;91:61–71. https://doi.org/10.1016/j.biopsych.2021.02.968.
Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Update on hypothetical model of Alzheimer’s disease biomarkers. Lancet Neurol. 2013;12:207–16. https://doi.org/10.1016/S1474-4422(12)70291-0.
Billings ME, Hale L, Johnson DA. Physical and Social Environment Relationship With Sleep Health and Disorders. Chest. 2020;157:1304–12. https://doi.org/10.1016/j.chest.2019.12.002.
Jackson CL, Powell-Wiley TM, Gaston SA, Andrews MR, Tamura K, Ramos A. Racial/Ethnic Disparities in Sleep Health and Potential Interventions Among Women in the United States. J Womens Health. 2002;2020(29):435–42. https://doi.org/10.1089/jwh.2020.8329.
van Mill JG, Hoogendijk WJG, Vogelzangs N, van Dyck R, Penninx BWJH. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry. 2010;71:239–46. https://doi.org/10.4088/JCP.09m05218gry.
Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, et al. Depression is Associated with Tau and Not Amyloid Positron Emission Tomography in Cognitively Normal Adults. J Alzheimers Dis JAD. 2020;74:1045–55. https://doi.org/10.3233/JAD-191078.
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3.
Dafsari FS, Jessen F. Depression-an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl Psychiatry. 2020;10:160. https://doi.org/10.1038/s41398-020-0839-1.
Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–50. https://doi.org/10.1001/jama.2017.3090.
Kang J, Tian Z, Wei J, Mu Z, Liang J, Li M. Association between obstructive sleep apnea and Alzheimer’s disease-related blood and cerebrospinal fluid biomarkers: A meta-analysis. J Clin Neurosci Off J Neurosurg Soc Australas. 2022;102:87–94. https://doi.org/10.1016/j.jocn.2022.06.004.
Luchsinger JA, Zetterberg H. Tracking the potential involvement of metabolic disease in Alzheimer’s disease-Biomarkers and beyond. Int Rev Neurobiol. 2020;154:51–77. https://doi.org/10.1016/bs.irn.2020.03.014.
Royse SK, Cohen AD, Snitz BE, Rosano C. Differences in Alzheimer’s Disease and Related Dementias Pathology Among African American and Hispanic Women: A Qualitative Literature Review of Biomarker Studies. Front Syst Neurosci. 2021;15:685957. https://doi.org/10.3389/fnsys.2021.685957.
Schultz SA, Boots EA, Almeida RP, Oh JM, Einerson J, Korcarz CE, et al. Cardiorespiratory Fitness Attenuates the Influence of Amyloid on Cognition. J Int Neuropsychol Soc JINS. 2015;21:841–50. https://doi.org/10.1017/S1355617715000843.
Gildner TE, Salinas-Rodríguez A, Manrique-Espinoza B, Moreno-Tamayo K, Kowal P. Does poor sleep impair cognition during aging? Longitudinal associations between changes in sleep duration and cognitive performance among older Mexican adults. Arch Gerontol Geriatr. 2019;83:161–8. https://doi.org/10.1016/j.archger.2019.04.014.
Acknowledgements
We would like to thank Jennifer Deberg, a medical librarian (blinded for review), at the University of Iowa Hardin Library for Health Sciences for assisting with the development of the search strategy for all web databases used for this study. We also thank Dr. Joel Geerling for advice and input on the qualities of the studies and clinical implications.
Funding
Part of this work was supported by the University of Iowa Institute for Clinical and Translational Science (NIH/ ACTS KL2TR002536, PI: Bassuk, Scholar: Moon), University of Iowa Center for Advancing Multimorbidity Science (NIH/ NINR P20 NR018081, MPI: Gardner & Rakel, Pilot PI: Moon), Alzheimer’s Association (AARG-19–618403). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/ACTS, NIH/ NINR, or Alzheimer’s Association.
Author information
Authors and Affiliations
Contributions
CM designed and developed this study. MZ, HD, KV screened the titles and abstracts of the searched articles and extracted data from the full text articles with guidance from CM. CM, MZ; KV drafted the introduction. All authors (AS, CM, MZ, YC, HD, KV) drafted the manuscript. All authors (AS, CM, MZ, YC, HD, KV) reviewed, revised, and approved the final draft for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moon, C., Schneider, A., Cho, YE. et al. Sleep duration, sleep efficiency, and amyloid β among cognitively healthy later-life adults: a systematic review and meta-analysis. BMC Geriatr 24, 408 (2024). https://doi.org/10.1186/s12877-024-05010-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-024-05010-4