Abstract
Orexins are hypothalamic neuropeptides that regulate several physiological functions, such as appetite, arousal, cognition, stress, sleep and metabolism. Emerging pieces of evidence suggest an orexinergic dysfunction in several neuropsychiatric disorders, including depression, anxiety and addiction. A syndromic overlap between behavioural variant frontotemporal dementia (bvFTD) and several psychiatric disorders was recently demonstrated. Therefore, we analysed cerebrospinal fluid (CSF) orexin A concentrations of 40 bvFTD and 32 non-demented patients, correlating neuropeptide concentrations with several clinical characteristics. A significant increase of orexin A concentrations was found in bvFTD patients when compared to controls (p<0.001). CSF orexin A concentration showed a correlation with Mini-Mental State Examination scores, drug assumption, history of compulsive behaviour and extrapyramidal signs. Moreover, we found a relationship between CSF markers of neurodegeneration, total tau and Aβ1–42 and CSF orexin A concentrations. Our study provides evidence of an orexinergic dysfunction in bvFTD, correlating with several clinical symptoms. Further larger studies are needed to confirm our data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioural variant frontotemporal dementia (bvFTD) is the most common clinical variant within the spectrum of frontotemporal lobar degeneration syndromes and is characterized by personality changes, apathy, decline in socially appropriate behaviour, and empathy. These symptoms reflect progressive deregulation of the neural circuits involved in social cognition, emotion regulation, motivation and decision making [1].
Orexins (orexin A and orexin B) are two neuropeptides synthesized by hypothalamic neurons with widespread projections throughout the central nervous system. Orexins regulate several physiological functions, like appetite, arousal, cognition, stress, sleep and metabolism. Several studies suggested a role for orexins in psychiatric disorders, including addiction, depression and anxiety [2]. Moreover, the modulation of the orexinergic system may also have a role in sleep disorders as well as rehabilitation of traumatic brain injury [3]. Recently, some studies evaluated cerebrospinal fluid (CSF) orexin levels in Alzheimer’s disease (AD), finding a high level of orexin A that correlates with AD progression, sleep fragmentation and neuropsychiatric symptoms [4].
Studies about orexins in bvFTD are few and not conclusive. A study with magnetic resonance imaging (MRI) of early-FTD patients and post-mortem analyses of bvFTD cases showed significant atrophy of the posterior hypothalamus with orexinergic neuron sparing [5]. In a small group of bvFTD patients, CSF orexin A concentrations were not significantly different from controls, but a negative correlation between neuropeptide concentrations and daytime somnolence was found [6]. Finally, a recent study showed that CSF pro-orexin levels are increased in FTD as well as in AD patients compared to controls [7].
Orexinergic system dysregulation could represent a neurobiological basis of certain clinical and behavioural features of patients with frontotemporal dementia. Thence, considering the above-mentioned observations, the purpose of this study was to better investigate the role of orexinergic system in bvFTD through the measurement of CSF orexin A concentrations and the evaluation of possible correlations with clinical symptoms.
Materials and methods
Patients
A group of 40 bvFTD consecutive patients (18 males and 22 females, mean age: 68.27 ± SD: 8.61 years), evaluated and treated at the Department of Neuroscience of University Hospital “Città della Salute e della Scienza” of Torino and at the Department of Neurology and Neurorehabilitation of “S. Giuseppe” Hospital, Piancavallo, Italy, were recruited for the study. Diagnosis of probable bvFTD was made according to Rascovsky et al. criteria [8]. For each patient, neuroimaging findings with magnetic resonance imaging (MRI) and positron emission tomography with 18fluorodeoxyglucose (18FDG-PET) were available. All patients underwent lumbar puncture with measurement of CSF levels of orexin A, Aβ1–42, p-tau and t-tau. Genetic variants in the MAPT, PGRN and C9orf72 genes were excluded. Cognitive functions were assessed by a standard neuropsychological evaluation, including Mini-Mental State Examination (MMSE). Detailed clinical history was recorded particularly focusing on cognitive and behavioural characteristics, drugs assumption and cardiovascular risk factors. A group of 32 cognitively healthy subjects (14 males and 18 females, mean age ± SD: 63.30 ± 14.21 years) examined for neurological conditions other than dementia (e.g. suspected polyneuropathy or multiple sclerosis) was used as controls. A complete description of the above-mentioned clinical procedures is available in the Supplementary materials.
CSF sample collection and biochemical analysis
All CSF samples were obtained by lumbar puncture, using an atraumatic needle, performed early in the morning (between 9.00 and 11.00 a.m.) after overnight fasting [9].
The CSF t-Tau, p-Tau181 and Aβ1-42 levels were measured separately, in duplicate, using commercially available sandwich enzyme-linked immunosorbent assays (ELISA) kits (Innnotest; Innogenetics/Fujirebio, Ghent, Belgium) according to the manufacturer’s instructions. Intra-assay and inter-assay coefficients of variation were 3.2% and 11.5% for t-tau, 1.7% and 11.4% for p-tau, 4.6% and 7.8% for Aβ1–42. The CSF Orexin levels were detected with the commercially available EIA kit (Phoenix Pharmaceuticals, Burlingame, CA, USA), based on the principle of competitive enzyme immunoassay. The intra-assay and inter-assay coefficients of variation were 7.5% and 5.8%, respectively. All samples were assayed in duplicate.
Statistical analysis
Continuous variables were described as mean and standard deviation or medians and range, and categorical ones as count and percentage. D’Agostino-Pearson’s test was used to assess the normality of included variables. Differences of continuous data between groups were studied by the unpaired t-test or its non-parametric variant (Mann-Whitney test), while differences of categorical variables by the Chi-square test with Yate’s correction. Correlation between continuous variables was analysed through Pearson or Spearman (in case of non-normal distribution) correlation. Multivariate generalized linear models (GLMs) were performed to estimate the quantitative relationship between CSF orexin A concentration and other variables considered as independent predictors. All analyses were run with R software (www.r-project.org). The level of statistical significance was defined at p < 0.05.
Results
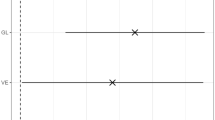
We found increased CSF orexin A concentrations in bvFTD patients (mean: 0.226 ng/mL ± SD: 0.103) compared to controls (mean: 0.139 ng/mL ± SD: 0.058), and this resulted statistically significant (p < 0.001) (Fig. 1), also when adjusted for age and sex (p < 0.001).
In addition, several clinical and demographic variables emerged as significantly linked to CSF orexin A concentrations in the bvFTD group (Table 1). We found a significant inverse correlation with MMSE, in both univariate and multivariate analyses (p < 0.001), while age at lumbar puncture and disease duration did not correlate with orexin A concentrations. In multivariate analysis, the presence of repetitive/compulsive behaviours as well as extrapyramidal signs was associated with increased CSF orexin A concentrations. Finally, neurodegenerative biomarkers of dementia, CSF t-Tau and Aβ1–42 concentrations co-variated with orexin A concentrations, though in the reciprocally opposite sense.
Discussion
In this study, we found that CSF orexin A concentrations are significantly increased in bvFTD patients in comparison to controls. In addition, we found that neuropeptide concentration correlated with several clinical characteristics of the disease, like repetitive/compulsive behaviour and extrapyramidal signs. Finally, a correlation between markers of neurodegeneration and CSF orexin A concentrations was found. Our data are in accordance with a previous study [7] showing increased CSF pro-orexin concentrations in a group of 32 FTD patients. On the contrary, Liguori and colleagues [6] found no difference in orexins concentrations comparing a small group of FTD patients (n = 11) with controls. However, this study was conducted in patients with a diagnosis of FTD syndrome, while our study included only bvFTD patients according to Rascovsky criteria.
Increased orexin A concentrations in bvFTD patients observed in our study may have several neurobiological explanations as well as important clinical implications. Interconnections between cortical areas and orexinergic neurons are increasingly under investigation, especially with concern to feeding and addiction disorders [10]. The prefrontal cortex emerges as a possible inhibitory interactor of hypothalamic orexinergic activity, and prefrontal disruption could mediate orexinergic tone deregulation, explaining higher orexin A concentrations in bvFTD patients.
We found an intriguing correlation between repetitive/compulsive behaviour and CSF orexin A concentrations. Several animal-model studies showed suppression of compulsive behaviours linked to abuse substances by orexin receptor antagonism [11]. Although pieces of evidence derived by human observational studies are fewer in number [12], we suggest a role of orexinergic system in enhancing motivation-linked compulsive behaviours, as they could be found in bvFTD patients. In addition, the presence of extrapyramidal signs showed to be an independent predictor of lower orexin A concentrations. This finding is consistent with previous pieces of evidence: in PD animal models orexin A demonstrated neuroprotective effects on dopaminergic neurons in substantia nigra [13], while orexinergic and dopaminergic neuronal loss was correlated with PD duration and progression in human autoptic cases [14]. Moreover, repeated CSF orexin measurements in PD patients revealed a decrease of levels over years and objective sleepiness correlated with decrease of CSF orexin levels [15], so suggesting that both orexin and dopamine deficiencies, and dopaminergic stimulation, may affect sleep and wakefulness in this neurodegenerative disorder [16, 17].
Finally, we found that CSF orexin A concentrations correlated with markers of neurodegeneration like Aβ1–42 and total tau. Preclinical and clinical studies suggested a role for Aβ1–42 in regulating amyloid clearance and, consequently, Alzheimer’s disease progression [18]. Furthermore, the negative correlation found with t-tau reinforces a role for this neuropeptide in neurodegenerative processes. Further studies are warranted in order to evaluate the potential role of orexin A as a biomarker of bvFTD.
Our study certainly has some limitations. For example, the low number of enrolled patients must be acknowledged. However, even if preliminary, correlations between orexin A concentrations and cognitive status, as well as motor and psychiatric symptoms, deserve additional investigations. To our knowledge, this is the largest clinical series demonstrating an increase of orexin A concentrations in cerebrospinal fluid of bvFTD patients. Moreover, it was possible to report several associations with the clinical characteristics of the disease. Better cognitive performance appeared to be a predictor of lower orexin A, thus reinforcing the hypothesis of a deregulation of this neuropeptide in the disease. Regarding specific neuropsychiatric manifestations, we found significant orexin A variations when independently assessing for a history of compulsive behaviours or extrapyramidal signs. In both cases, a possible explanation is based on orexinergic physiological functions and interconnections with other neurotransmitter systems. Finally, also neurodegenerative process, as delineated by CSF markers, emerged as an independent predictor of orexin A variability in this study, suggesting a complex role of neuronal loss in deregulating the cognitive-behavioural functions related to orexinergic system.
Abbreviations
- bvFTD:
-
Behavioural variant frontotemporal dementia
- AD:
-
Alzheimer disease
- PD:
-
Parkinson’s disease
References
Bang J, Spina S, Miller BL (2015) Frontotemporal dementia. Lancet 386:1672–1683
Chen Q, De Lecea L, Hu Z, Gao D (2015) The hypocretin/orexin system: an increasingly important role in Neuropsychiatry. Med Res Rev 35:1–46
Tang H, Zhu Q, Li W, Qin S, Gong Y, Wang H, Shioda S, Li S, Huang J, Liu B, Fang Y, Liu Y, Wang S, Guo Y, Xia Q, Guo Y, Xu Z (2019) Neurophysiology and treatment of disorders of consciousness induced by traumatic brain injury: orexin signaling as a potential therapeutic target. Curr Pharm Des 25:4208–4220
Liguori C, Mercuri N, Nuccetelli M, Izzi F, Bernardini S, Placidi F (2018) Cerebrospinal fluid orexin levels and nocturnal sleep disruption in Alzheimer’s disease patients showing neuropsychiatric symptoms. J Alzheimers Dis 66(3):993–996
Piguet O, Petersén A, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, Halliday GM (2011) Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol 69:312–319
Liguori C, Romigi A, Mercuri NB, Nuccetelli M, Izzi F, Albanese M, Sancesario G, Martorana A, Sancesario GM, Bernardini S, Marciani MG, Placidi F (2014) Cerebrospinal-fluid orexin levels and daytime somnolence in frontotemporal dementia. J Neurol 261:1832–1836
Heywood WE, Hallqvist J, Heslegrave AJ, Zetterberg H, Fenoglio C, Scarpini E, Rohrer JD, Galimberti D, Millsa K (2018) CSF pro-orexin and amyloid-β38 expression in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 72:171–176
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477
Doherty CM, Forbes RB (2014) Diagnostic lumbar puncture. Ulster Med J 83(2):93–102
Mena JD, Selleck RA, Baldo BA (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33(47):18540–18552
Han Y, Yuan K, Zheng Y, Lu L (2020) Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull 36(4):432–448
Chen WY, Kao CF, Chen PY, Lin SK, Huang MC (2016) Orexin-A level elevation in recently abstinent male methamphetamine abusers. Psychiatry Res 239:9–11
Liu MF, Xue Y, Liu C, Liu YH, Diao HL, Wang Y, Pan YP, Chen L (2018) Orexin-A exerts neuroprotective effects via OX1R in Parkinson’s disease. Front Neurosci 15(12):835
Thannickal TC, Lai YY, Siegel JM (2007) Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130:1586–1595
Wienecke M, Werth E, Poryazova R, Baumann-Vogel H, Bassetti CL, Weller M, Waldvogel D, Storch A, Baumann CR (2012) Progressive dopamine and hypocretin deficiencies in Parkinson’s disease: is there an impact on sleep and wakefulness? J Sleep Res 21(6):710–717
Zibetti M, Romagnolo A, Merola A, Priano L, Montanaro E, Angrisano S, Tribolo A, Cicolin A, Lopiano L (2017) A polysomnographic study in parkinsonian patients treated with intestinal levodopa infusion. J Neurol 264(6):1085–1090
Videnovic A, Golombek D (2013) Circadian and sleep disorders in Parkinson’s disease. Exp Neurol 243:45–56
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 13;326(5955):1005-7.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards statements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest
The study was conducted in accordance with the Declaration of Helsinki. All participants gave their informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 25 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roveta, F., Marcinnò, A., Cremascoli, R. et al. Increased orexin A concentrations in cerebrospinal fluid of patients with behavioural variant frontotemporal dementia. Neurol Sci 43, 313–317 (2022). https://doi.org/10.1007/s10072-021-05250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05250-x