Abstract
Background
Fingolimod, an oral sphingosine 1-phosphate receptor modulator, is approved by EMA for relapsing-remitting multiple sclerosis (RRMS).
Objectives
To assess the effectiveness and safety of fingolimod in patients with RRMS in real-world clinical practice in Portugal.
Methods
Retrospective, multicentre, non-interventional study, reporting 3 years follow-up of data collected from October 2015 to July 2016. Sociodemographic data and previous treatments at baseline and data regarding disease evolution, including number of relapses, annualised relapse rates (ARR) and Expanded Disability Status Scale (EDSS), were collected.
Results
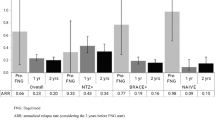
Two-hundred and seventy-five participants were enrolled in the REALMS study. Results showed that the main reason to switch to fingolimod was failure of previous treatment (56.7%) and only 3.6% were naïve patients. In the total population, there was a significant decrease in ARR of 64.6% in the first year of treatment, 79.7% in the second year and 82.3% in the third year, compared with baseline. More than 67.0% of patients had no relapses during the 3 years after switching to fingolimod. EDSS remained stable throughout the study.
Conclusions
Therapy with fingolimod showed a sustained effectiveness and safety over the 3 years, particularly on patients switched from first-line drugs (BRACE). No new safety issues were reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fingolimod belongs to a class of drugs that targets the sphingolipid-regulated signaling system, acting as a functional antagonist of the sphingosine-1-phosphate type 1 (S1P1) receptor immunomodulatory [1], although some authors consider it to be an immunosuppressant [2]. It is a prodrug that is phosphorylated by sphingosine kinases to its active form, phosphofingolimod [1]. S1P1 is expressed abundantly on T and B lymphocytes, and fingolimod induces its downregulation by sequestering T cells in lymph nodes. This action prevents these cells from infiltrating inflammatory lesions in the central nervous system (CNS) [3], and fingolimod has been shown to decrease the pro-inflammatory marker IL-6 [4].
Fingolimod has shown to be effective in all four key measures of disease activity (relapse rate, disability progression, magnetic resonance imaging activity, and brain volume loss) compared with placebo or intramuscular interferon beta-1a in three pivotal clinical trials [5,6,7], although no differences were detected in the time to the confirmed progression of disability in the TRANSFORMS study, given its short duration (12 months) [6].
Fingolimod (Gilenya®) is the first approved oral disease-modifying therapy (DMT) worldwide. In the European Union [8], as well as in Portugal [9], fingolimod was approved as a single DMT in adult and paediatric patients aged 10 years and older [10] with highly active relapsing-remitting multiple sclerosis (RRMS) despite a full and adequate course of treatment with at least one DMT or patients with rapidly evolving severe RRMS defined by two or more disabling relapses in one year, and with one or more gadolinium-enhancing lesions on brain magnetic resonance imaging (MRI) or a significant increase in T2 lesion load as compared with a previous recent MRI.
Several studies of fingolimod in real-world populations have been published [11,12,13,14,15] confirming its effectiveness and safety shown in clinical trials in a more heterogeneous population.
The aim of the present study was to provide further data on the effectiveness and safety of fingolimod in a real-world clinical setting, for treatment-naïve and pre-treated patients with RRMS in Portugal.
Methods
Study design
REALMS was a retrospective, multicentre, national non-interventional study. Medical records were used to collect real-world evidence for the effectiveness and safety of fingolimod, as well as demographic and clinical characteristics of these patients. The study was conducted according to the tenets of the Declaration of Helsinki in its latest amendment (Brazil, 2013) and was approved by the Ethics Committee of each participating centre.
Setting and participants
REALMS was a Portuguese multicentre study that retrospectively collected real-world evidence on the effectiveness, tolerability and safety from the clinical records of RRMS patients under fingolimod treatment in the most representative Portuguese MS centres. This paper reports 3-year follow-up of data collected from the 9 participating centres throughout Portugal from October 2015 to July 2016.
Inclusion criteria were as follows: age 18 years or more; diagnosis of RRMS according to the McDonald Criteria from 2010 [16]; patients who had initiated treatment with fingolimod at least 12 months before study enrolment, including those previously treated with interferon-β and/or glatiramer acetate, natalizumab or treatment naïve; at least 12 months of follow-up after initiating fingolimod treatment and sufficient data available on the clinical files. Treatment could have been discontinued, either temporarily or permanently, during these 12 months of follow-up (in case of temporary discontinuation, date of treatment initiation has been considered the date of the first administration of fingolimod); and patients that accepted to participate in the study and provided written informed consent to collect and analyse their data.
Exclusion criteria were patients who had previously been treated with fingolimod in a clinical trial before the inclusion in this study and had progressive course of MS (either secondary or primary progression) at the date of fingolimod treatment initiation.
Collected variables and definitions
All variables were collected on an eCRF specifically designed for the study. The following data were obtained for all patients at baseline (12 months after initiation of fingolimod): age, sex, disease duration, date of first relapse, prevalence of other symptoms of MS, previous DMTs, duration of previous treatment with interferon-β or glatiramer acetate or natalizumab, reasons to switch to fingolimod, annualised relapse rates (ARR) and Expanded Disability Status Scale (EDSS) before switching to fingolimod, relevant comorbidities, and concomitant treatments. At follow-up, the following variables were collected: number of relapses, days to first relapse, ARR, EDSS, relevant comorbidities, treatment with costicosteroids, adverse events (AEs), fingolimod discontinuation, when applicable, and reason for discontinuation. A relapse was defined as patient-reported symptoms or objectively observed signs typical of an acute inflammatory demyelinating event in the CNS, current or historical, with duration of at least 24 hours, in the absence of fever or infection [16]. Progression of disability was defined as a 1-point increase in the EDSS score (or a half-point increase for patients with a baseline score above 5.0) that was confirmed at 6 months for up to 24 months [17].
Quantitative variables and groups
In addition to descriptive statistics, inference statistics was performed in the whole population and three subgroups depending on treatment previous to fingolimod: naïve, interferon-β/glatiramer acetate or natalizumab. ARR and EDSS in the previous year and during the first 12, 24 and 36 months of treatment with fingolimod were compared. The proportion of relapse-free patients was also compared between these time points. The difference between these variables over time, within the same group, was also assessed.
Safety assessments
The safety assessments analysed were as follows: descriptives of AEs during the first 24 h of the first fingolimod administration; description of AEs occurring after fingolimod treatment initiation; and maintenance of treatment during the first year of treatment with fingolimod. Disease activity, e.g. relapses or progression, were considered AEs.
Statistical methods
An intent-to-treat (ITT) statistical analysis was performed. According to the original statistical analysis plan (SAP), statistical analysis was performed with available data, and no method of imputation for missing data was used. Quantitative variables were tested for normality using the Shapiro-Wilk test. Since the majority of quantitative variables were not normally distributed, baseline median, interquartile range (IQR), minimum and maximum are presented. For categorical variables, number and percentage of total are presented. Within-group analyses for quantitative variables were performed using the Friedman test and adjusted for multiple comparisons using the Sidak correction. For categorical variables, Cochran’s Q statistics or McNemar’s test were used as appropriate. The Kendall’s W test was used to analyse correlations. Between-group analyses for quantitative variables were performed using the Kruskal-Wallis test and adjusted for multiple comparisons using the Sidak correction. For categorical variables, the χ2 test was used. The Spearman ρ or Kendall’s τ-b were used to analyse correlations as appropriate. Predictors of EDSS at 3 years and ARR at 1, 2 and 3 years after switching to fingolimod were analysed using multivariate regressions using the backward conditional method. The dependent variable on the EDSS model was EDSS at 3 years, and the independent variables were sex, age, ARR in the year before switching to fingolimod, number of previous DMTs and years until switch to fingolimod. On all ARR models, the independent variables were the same as the ones included in the EDSS model plus: (1) baseline EDSS for the dependent variable ARR at 1 year; (2) baseline EDSS and ARR at 1 year for the dependent variable ARR at 2 years; and (3) baseline EDSS, ARR at 1 year and ARR at 2 years for the dependent variable ARR at 3 years. EDSS at baseline was not included in the predictor model of EDSS since these variables were significantly correlated (n = 37, Spearman ρ = 0.754, p = 0.01). A significance level of α = 0.05 was used (two sided). The software used was the SPSSv20.0 statistical package.
Results
Patient population
The REALMS study included 275 participants. All tables and figures state the number of patients with data available for each analysed variable and each time point. Missing data were considered to be missing completely at random (MCAR). Forty-one (14.9%) patients discontinued treatment with fingolimod during the study, twenty-seven (9.8%) of which permanently and fourteen (5.1%) temporarily. Therefore, the overall attrition rate of enrolled patients was 9.8%.
Demographics and clinical baseline characteristics
Demographic and clinical characteristics of the total population and the three subgroups are described in Table 1. The 13 patients missing from Table 1 had previous treatments other than BRACE or natalizumab (off-label azathioprine (n = 4), mitoxantrone (n = 4), Ig IV (n = 4) and methotrexate (n = 1)).
The majority of patients were women (~ 65%). The median age at diagnosis for the total cohort and subgroups was between 40.0 and 43.0 years old, with the exception of the naïve subgroup who was younger (36.5 years). The median (IQR) disease duration was 10.0 (9.0) years. As expected, the naïve sub-group presented significant less years of disease duration compared with the interferon-β/glatiramer acetate and natalizumab sub-groups (4.5 vs. 10.0 and vs. 12.0 years, respectively, p < 0.05).
Reasons to switch to fingolimod
The reasons to switch to fingolimod therapy were, in decreasing order of frequency, failure of previous treatment (56.7%), natalizumab withdrawal due to progressive multifocal leukoencephalopathy risk (35.3%) or other, such as adverse events from other treatments (6.2%). The remaining 3.6% were naïve patients with rapid disease progression, who initiated fingolimod as first-line therapy.
Annualised relapse rate
Considering the total population, there was a 64.6% decrease in ARR (0.79 vs. 0.28, p < 0.001) in the first year of treatment (Fig. 1). ARR further decreased by 79.7% in year 2 and 82.3% in year 3 compared with baseline (0.79 vs. 0.16 and vs. 0.14, respectively, p < 0.001). Patients previously treated with interferon-β or glatiramer acetate therapies showed an ARR reduction of 76.3% from baseline to year 1 post-fingolimod treatment (0.93 vs. 0.22, p < 0.001). The mean ARR decreased by 87.1% in year 2 (0.12, p < 0.001) and 84.9% in year 3 (0.14, p < 0.001) compared with baseline. Patients previously treated with natalizumab (n = 83) did not show a significant reduction of ARR in the first year of treatment. However, in these patients, ARR decreased by 48.8% in year 2 (0.43 vs. 0.22, p < 0.002) and 62.7% in year 3 (0.43 vs. 0.16, p < 0.001) compared with baseline.
Therapy with fingolimod showed a sustained effectiveness over the 3 years.
Expanded Disability Status Scale
The median baseline EDSS in the whole REALMS population was 3.00, changed to 2.50 after 1 year of fingolimod treatment (p > 0.05) and remained stable up to the end of the 3-year follow-up (Fig. 2a). When analysing by previous DMTs, both the previous interferon-β or glatiramer acetate and previous natalizumab groups remained with a stable EDSS over the 3 years of fingolimod therapy (Fig. 2b). Between group analyses showed that the interferon-β or glatiramer acetate group presented a significantly lower median EDSS compared with natalizumab 1 year after fingolimod therapy (p < 0.05), with both sub-groups remaining stable without differences between them after 2 and 3 years of fingolimod therapy.
Relapse-free patients
Considering all patients of the REALMS study, 78.9 to 90.5% were relapse free in each of the 3 years of this study. From years 1 to 2, relapse-free patients significantly increased (78.9 vs. 87.3%, p = 0.016) and from years 1 to 3, further increased to 90.5% (p < 0.001). More than 67.0% of patients had no relapses during the 3 years after switching to fingolimod.
Adverse events
There were a total of 61 (22.2%) AEs in 49 (17.8%) patients (Table 2). AEs were mostly related to disease activity (5.5%), followed by reductions in lymphocyte counts, opportunistic infections, increased liver enzymes and hypertension. None of these AEs were classified as serious AEs (SAEs).
Multivariate regression analyses
Predictors of EDSS at 3 years and ARR at 1, 2 and 3 years after switching to fingolimod were analysed with multivariate regressions using the backward conditional method (Table 3). Age in years and years until switch to fingolimod were positive predictors of EDSS at 3 years. Prior number of DMTs was a positive predictor of ARR at 1 year, and ARR at 1 year was a positive predictor of ARR at 2 years. None of the variables was a predictor of ARR at 3 years.
Discussion
This study aimed to characterise the patients’ profile treated with fingolimod in the Portuguese real-word clinical practice, as well as to assess its effectiveness and safety.
In Portugal, the effectiveness and safety of fingolimod in a real-word population was previously studied in two single-centre studies [11, 18]. A multicentric study was necessary to contribute to the validation of the reported data. In these two studies, the reported discontinuation rate was 10.6% [11] and 15.6% (at 12 months) [18]. These attrition rates are similar to the ones found in the present study (9.8%), which are also similar to the ones reported by the real-word study with fingolimod from UK (approximately 8%) [13]. In other two real-world studies, one conducted in the Czech Republic, the GOLEMS study, 11.3% of patients discontinued fingolimod at or before 12 months [19] and another one conducted in Spain, the MS NEXT, 3.9% of patients permanently discontinued fingolimod during the first year of treatment [20].
Overall, the proportion of relapse-free patients significantly increased from 78.9 to 90.5% from the first to the third year after switching to fingolimod therapy. Moreover, 67% of the patients had no relapses during the 3 years after switching to fingolimod. These results are in accordance with the findings by Mazibrada et al and the MS NEXT study that reported 83.7% relapse-free patients 12 months after fingolimod initiation [13], and 67% relapse-free patients after two years of fingolimod treatment [20], respectively. The GENIUS study concluded that fingolimod appeared to be effective in naïve patients and after first-line treatment failure in reducing risk of relapse and disease activity throughout a 2-year follow-up [21]. In the two Portuguese real-world studies, the percentage of relapse-free patients at year 1 after switching to fingolimod was 75% [11] and 80.4% [18]. However, in this last study, the percentage of relapse-free patients decreased from years 1 to 3 of fingolimod treatment. Nevertheless, the percentage of patients with no relapses during the 3 years of fingolimod treatment was 60.8% [18], similar to that observed in our study.
Considering the total population and the group switching from interferon-β/glatiramer acetate, ARR significantly decreased in the first year of treatment with fingolimod and remained lower than baseline over 3 years. These results are in accordance with those reported by the PANGAEA study after 5 years of fingolimod therapy [22], a study conducted with similar methodological characteristics as the current study [23]. These results are also consistent with previously reported Portuguese studies whose results also showed a decrease in ARR in the first year of treatment with fingolimod [11, 18] and with the MS NEXT study that reported a 76% decrease in ARR after 2 years of fingolimod [20].
Mazibrada et al. reported that ARR in patients switching from natalizumab to fingolimod significantly decreased after the first year of fingolimod treatment [13], and the PANGAEA study at 4 years showed a decrease in ARR after the first year of fingolimod treatment that was maintained through 4 years, compared with baseline [24]. Our results showed that in the group of patients switching from natalizumab, there was no reduction of ARR in the first year of fingolimod treatment, although ARR decreased in years 2 and 3 compared with baseline. These results are in line with the ones reported by the two Portuguese real-world studies [11, 18].
Regarding disability, and as observed in the majority of studies [22, 25,26,27], the median value of EDSS at baseline was 3.0. Within-group analyses over time showed that EDSS remained stable over the 3 years after switching to fingolimod regardless of previous interferon-β/glatiramer acetate therapies. These results are in line with the FREEDOMS II and TRANSFORMS trials [6, 28]. Recently published results of real-world studies also showed a stable EDSS after switching to fingolimod, regardless of previous therapy [11, 13, 18, 22, 24].
Disability progression independent of relapse activity (PIRA) has been described as a frequent phenomenon in patients classified as RRMS. As a matter of fact, the term silent progression was recently proposed to describe the insidious disability that accrues in many patients who satisfy traditional criteria for RRMS. Therefore, we think that these results reflect precisely this phenomenon, since not all patients included in this cohort fulfil the criteria for secondary progressive MS. This suggests that the same process that underlies SPMS likely begins far earlier than it is generally recognised and supports a unitary view of MS biology, with both focal and diffuse tissue destructive components, and with inflammation and neurodegeneration occurring throughout the disease spectrum [29].
Interestingly, between-group analysis showed that the interferon-β/glatiramer acetate group presented a significantly lower median EDSS compared with natalizumab 1 year after fingolimod therapy and remained stable over the ensuing 2 years. These results have not been shown in the FREEDOMS, FREEDOMS II and TRANSFORMS trials [5, 6, 28], and are most probably a consequence from the fact that most patients switched to fingolimod based on clinical activity (relapses) in close temporal association with the time of switch. In line with this hypothesis, EDSS decreases were mostly due to switches from interferon-β/glatiramer acetate and not observed in the group that switched from natalizumab, in which the primary reason for the switch was not lack of efficacy, but being seropositive for the John Cunningham virus.
On multivariate regression analysis, the higher the age and the years until switch to fingolimod the higher the EDSS after 3 years. Also, the higher the number of previous DMTs the higher the ARR at year 1 after fingolimod initiation, and the higher the ARR at 1 year the higher the ARR at 2 years after fingolimod initiation. Taken together, and from a clinical standpoint, the faster the switch to fingolimod the better the results for the patient.
A sensitivity analysis for EDSS predictors at 3 years considering only patients that completed the 3-year follow-up (n = 52) has been performed. Age remained a positive predictor, although with slightly different values (Exp(B), 1.111; 95% CI, 1.043–1.183; p = 0.002), years until switch to fingolimod was no longer a predictor of EDSS at 3 years but prior DMTs were (Exp(B), 1.528; 95% CI, 1.021–2.289; p = 0.040). In fact, these two variables have the same interpretation and the conclusion to be drawn is the same: the early treatment with fingolimod is the real predictor of EDSS at 3 years, regardless if it is measured by proxy in years until switch to fingolimod or in prior DMTs. Therefore, this sensitivity analysis strengthens our results and conclusions.
In line with trials and real-world data, we observed no significant differences in ARR 1 year after switching from natalizumab to fingolimod whereas when changing from previous interferon-β/glatiramer acetate, the improvement of ARR at 1 year was evident.
In our population, and similar to other studies [11, 15, 18, 19, 30], fingolimod has shown to have a good safety profile. The proportion of patients discontinuing treatment was mostly related to disease activity (5.5%).
In RRMS, inflammation and the consequent presence of lymphocytes in the CNS is a hallmark of the disease, occurring in all its stages and courses. Therefore, new therapies that could act as promoters of the redistribution of lymphocytes back into circulation may reduce the immunomodulated axonal attack [31]. Thereby, previous treatment could hinder baseline parameters of inflammatory activity and future relapses induced by specific treatment. Perhaps this explains our results that showed that the time until switch to fingolimod was a predictor of higher EDSS at 3 years and a higher number of previous DMTs was a predictor of higher ARR at year 1 after fingolimod initiation.
Limitations and strengths
REALMS had the inherent limitations of secondary data collection. There were too many missing MRI data to allow for an analysis of these parameters. Also, and although all investigators have been asked to record all adverse events, this is impossible to guarantee. One of the biggest strengths of this study was the large sample size. This was the largest observational study done up to date, involving 9 of the most important MS centres in Portugal.
Conclusions
The REALMS study provides real-world data confirming the effectiveness and safety of fingolimod under real-world conditions, in the Portuguese population, consistent with phase 3 trials. Fingolimod is an effective treatment in real-world populations with RRMS regardless of previous DMTs. Moreover, significant reductions in relapses were seen after switching from interferon-β or glatiramer acetate to fingolimod, suggesting that these patients benefit from switching to fingolimod. Fingolimod revealed to be a good option treatment for the majority of patients after switch from natalizumab, since most of these patients were relapse free (88.9%) and free from progression of disability (66.7%) after 3 years of treatment with fingolimod. Furthermore, these data suggest that the earlier the treatment with fingolimod, the better the outcomes for patients with RRMS.
Change history
09 November 2020
A Correction to this paper has been published: https://doi.org/10.1007/s10072-020-04879-4
References
La Mantia L, Tramacere I, Firwana B, Pacchetti I, Palumbo R, Filippini G (2016) Fingolimod for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 4:CD009371
AlSharoqi IA, Aljumah M, Bohlega S, Boz C, Daif A, El-Koussa S, Inshasi J, Kurtuncu M, Müller T, Retief C, Sahraian MA, Shaygannejad V, Slassi I, Taha K, Zakaria M, Sørensen PS (2020) Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A Narrative Review. Neurol Ther
Chun J, Hartung HP (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33:91–101
Dalla Costa G, Finardi A, Garzetti L, Carandini T, Comi G, Martinelli V, Furlan R (2018) Disease-modifying treatments modulate myeloid cells in multiple sclerosis patients. Neurol Sci 39:373–376
Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362:387–401
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L (2010) Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362:402–415
Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD (2014) Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 13:545–556
Fingolimod SPC. Approved by EMA. Updated 2017
Direcção Geral de Saúde Portugal (2015) Norma de Orientação Clínica sobre Terapêutica Modificadora da Esclerose Múltipla em Idade Pediátrica e no Adulto
Chitnis T, Arnold DL, Banwell B, Brück W, Ghezzi A, Giovannoni G, Greenberg B, Krupp L, Rostásy K, Tardieu M, Waubant E, Wolinsky JS, Bar-Or A, Stites T, Chen Y, Putzki N, Merschhemke M, Gärtner J (2018) Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 379:1017–1027
Correia I, Batista S, Marques IB, Sousa M, Ferreira R, Nunes C, Macario MC, Sousa L (2016) The effectiveness of fingolimod in a Portuguese real-world population. Mult Scler Relat Disord 6:41–48
Izquierdo G, Damas F, Paramo MD, Ruiz-Pena JL, Navarro G (2017) The real-world effectiveness and safety of fingolimod in relapsing-remitting multiple sclerosis patients: an observational study. PLoS One 12:e0176174
Mazibrada G, Sharples C, Perfect I (2018) Real-world experience of fingolimod in patients with multiple sclerosis (MS Fine): An observational study in the UK. Mult Scler J Exp Transl Clin 4:2055217318801638
Zecca C, Roth S, Findling O, Perriard G, Bachmann V, Pless ML, Baumann A, Kamm CP, Lalive PH, Czaplinski A (2018) Real-life long-term effectiveness of fingolimod in Swiss patients with relapsing-remitting multiple sclerosis. Eur J Neurol 25:762–767
Laroni A, Brogi D, Brescia Morra V, Guidi L, Pozzilli C, Comi G, Lugaresi A, Turrini R, Raimondi D, Uccelli A, Mancardi GL (2017) Safety and tolerability of fingolimod in patients with relapsing-remitting multiple sclerosis: results of an open-label clinical trial in Italy. Neurol Sci 38:53–59
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 33:1444–1452
Ribeiro de Barros AH, Fiadeiro Sequeira JP, Lopes de Sousa AS, Chegancas Capela CM, Gomes Pedrosa RM, Dos Santos Manita MA (2018) Safety and Effectiveness of Fingolimod in Real-World Multiple Sclerosis Portuguese Patients. Clin Neuropharmacol 41:129–135
Ticha V, Kodym R, Pocikova Z, Kadlecova P (2017) Real-World outcomes in fingolimod-treated patients with multiple sclerosis in the Czech Republic: results from the 12-month GOLEMS study. Clin Drug Investig 37:175–186
Mallada-Frechin J, Meca-Lallana V, Barrero F, Martinez-Gines ML, Marzo-Sola ME, Ricart J, Garcia E En Representacion de Los Investigadores Del Estudio Ms Next E (2018) [Efficacy and safety of fingolimod in routine clinical practice in patients with relapsing-remitting multiple sclerosis in Spain: an intermediate analysis of the MS NEXT study]. Rev Neurol 67:157–167
Comi G, Pozzilli C, Morra VB, Bertolotto A, Sangalli F, Prosperini L, Carotenuto A, Iaffaldano P, Capobianco M, Colombo D, Nica M, Rizzoli S, Trojano M (2020) Effectiveness of fingolimod in real-world relapsing-remitting multiple sclerosis Italian patients: the GENIUS study. Neurol Sci
Ziemssen T, Albrecht H, Haas J, Klotz L, Lang M, Lassek C, Schmidt S, Tackenberg B, Cornelissen C (2017) 5 years effectiveness of fingolimod in daily clinical practice: results of the non-interventional study PANGAEA documenting RRMS patients treated with fingolimod in Germany. Poster P6345 presented at the 69th American Academy of Neurology (AAN) annual meeting, April 22 – 28, 2017, Boston
Ziemssen T, Kern R, Cornelissen C (2015) The PANGAEA study design - a prospective, multicenter, non-interventional, long-term study on fingolimod for the treatment of multiple sclerosis in daily practice. BMC Neurol 15:93
Ziemssen T, Albrecht H, Haas J, Klotz L, Lang M, Lassek C, Schmidt S, Tackenberg B, Cornelissen C (2016) 4 years PANGAEA: long term data on effectiveness and safety from natalizumab patients switching to fingolimod in real world. Poster P6188 presented at the 68th American Academy of Neurology (AAN) annual meeting, April 15 – 21, 2016, Vancouver
Al-Hashel J, Ahmed SF, Behbehani R, Alroughani R (2014) Real-world use of fingolimod in patients with relapsing remitting multiple sclerosis: a retrospective study using the national multiple sclerosis registry in Kuwait. CNS Drugs 28:817–824
Ouspid E, Razazian N, Moghadasi AN, Moradian N, Afshari D, Bostani A, Sariaslani P, Ansarian A (2018) Clinical effectiveness and safety of fingolimod in relapsing remitting multiple sclerosis in Western Iran. Neurosciences (Riyadh) 23:129–134
Ziemssen T, Albrecht H, Haas J, Klotz L, Lang M, Lassek C, Schmidt S, Tackenberg B, Cornelissen C (2016) 4 years PANGAEA: A 5-year non-interventional study of safety, effectiveness and pharmacoeconomic data for fingolimod patients in daily clinical practice – effectiveness update. Poster P3072 presented at the 68th American Academy of Neurology (AAN) annual meeting, April 15 – 21, 2016, Vancouver
Kappos L, O'Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K, Ritter S, Schlosshauer R, von Rosenstiel P, Zhang-Auberson L, Francis G (2015) Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology. 84:1582–1591
Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, Caverzasi E, Bischof A, Gundel T, Zhu AH, Papinutto N, Stern WA, Bevan C, Romeo A, Goodin DS, Gelfand JM, Graves J, Green AJ, Wilson MR, Zamvil SS, Zhao C, Gomez R, Ragan NR, Rush GQ, Barba P, Santaniello A, Baranzini SE, Oksenberg JR, Henry RG, Hauser SL (2019) Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 85:653–666
Ziemssen T, Albrecht H, Haas J, Klotz L, Lang M, Lassek C, Schmidt S, Tackenberg B, Cornelissen C (2017) 5 years safety of fingolimod in real world: First results from PANGAEA, a noninterventional study of RRMS patients treated with fingolimod, on safety and adherence after 5 years of fingolimod in daily clinical practice. Poster P5365 presented at the 69th American Academy of Neurology (AAN) annual meeting, April 22 – 28, 2017, Boston
Langer-Gould A, Popat RA, Huang SM, Cobb K, Fontoura P, Gould MK, Nelson LM (2006) Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol 63:1686–1691
Acknowledgments
The authors would like to thank all study personnel who participated in the collection of these data. We thank Constança Coelho PhD, from x2 Science Solutions, for editorial assistance. The authors had full control of the content and made the final decision on all aspects of this article.
Funding
This study was funded by Novartis Pharma, Portugal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sónia Batista has received compensation as a speaker in meetings and for consulting and advisory board participation from Biogen, Bayer, Merck Serono,Novartis, Roche, and Sanofi-Genzyme.
Ana Martins da Silva has received compensation as a speaker in meetings and for consulting, clinical trial research, and advisory board participation fromBiogen, Bayer, Merck Serono, Novartis, Roche, and Sanofi-Genzyme.
Lívia Sousa has received compensation as a speaker in meetings and for consulting, clinical trial research, and advisory board participation from Biogen,Bayer, Merck Serono, Novartis, Roche, and Sanofi-Genzyme.
Ethical approval
The study protocol was approved by the local Ethics Committee. An informed consent was obtained from each participant and the research was conducted according to the principles of the Helsinki Declaration.
Informed consent
An informed consent was obtained from each participant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Batista, S., Nunes, C.C., Cerqueira, J.J. et al. REALMS study: real-world effectiveness and safety of fingolimod in patients with relapsing-remitting multiple sclerosis in Portugal. Neurol Sci 42, 1995–2003 (2021). https://doi.org/10.1007/s10072-020-04726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04726-6