Abstract
Background
Multiple sclerosis (MS) is an autoimmune, neuroinflammatory, and neurodegenerative disease of the central nervous system. B cells have recently emerged as a promising target to significantly reduce inflammatory disease activity in MS, with successful trial studies using antiCD20 therapies. However, real-life data about safety and efficacy are limited.
Objectives
To analyze the clinical and radiological inflammatory activity, adherence to therapy, and safety of rituximab (RTX) in an MS patients’ sample, treated from 2015 to 2018 in our center
Patients and methods
Retrospective study on prospectively collected data about relapses, disability progression, and radiological activity (new T2 lesions and Gd-enhancing lesions) were recorded and used to assess no evidence of disease activity (NEDA) at 12 months. RTX-related adverse events were recorded. RTX was administered intravenously at a dosage of 1000 mg twice 2 weeks apart, then every 6 months.
Results
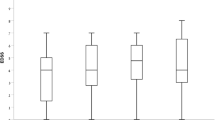
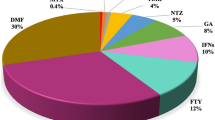
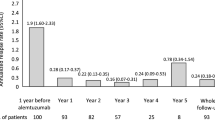
Sixty-nine patients were included. Fifty-three (76.8%) had a relapsing-remitting, two a primary progressive course, and 14 a secondary progressive course. The mean follow-up period was 16 ± 9.7 months. Thirty-five (50.7%) patients had relapses in the year prior to RTX therapy, with a mean annualized relapse rate of 0.75, significantly reduced to 0.36 at 12 months (p < 0.001). Among the 36 patients included in the study who had an MRI available at 12 months, MRI activity was reduced from 88% (n = 32) to 8.3% (n = 3) at follow-up (p < 0.001). Twelve (17.4%) patients suspended RTX during the study.
Conclusions
Our real-life experience confirms that off-label therapy with RTX may represent a valid, cost-effective therapeutic option in MS.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, MPA, upon reasonable request.
References
Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, HERMES Trial Group (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358:676–688. https://doi.org/10.1056/NEJMoa0706383
Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, Trinkaus K, Song SK, Cross AH (2010) Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 74:1860–1867. https://doi.org/10.1212/WNL.0b013e3181e24373
Bar-Or A, Calabresi PAJ, Arnlod D et al (2008) Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 63:395–400. https://doi.org/10.1002/ana.21363
Alcalá C, Gascón F, Pérez-Miralles F, Gil-Perotín S, Navarré A, Boscá I, Coret F, Casanova B (2018) Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol 265:1690–1697. https://doi.org/10.1007/s00415-018-8899-3
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, Islam-Jakobsson P, Malmeström C, Axelsson M, Vågberg M, Sundström P, Lycke J, Piehl F, Svenningsson A (2016) Rituximab in multiple sclerosis. Neurology. 87:2074–2081. https://doi.org/10.1212/WNL.0000000000003331
D’Amico E, Zanghì A, Chisari CG et al (2019) Effectiveness and safety of rituximab in demyelinating diseases spectrum: an Italian experience. Mult Scler Relat Disord 27:324–326. https://doi.org/10.1016/j.msard.2018.09.041
Spelman T, Frisell T, Piehl F, Hillert J (2018) Comparative effectiveness of rituximab relative to IFN-β or glatiramer acetate in relapsing-remitting MS from the Swedish MS registry. Mult Scler J 24:1087–1095. https://doi.org/10.1177/1352458517713668
Scotti B, Disanto G, Sacco R, Guigli M’, Zecca C, Gobbi C (2018) Effectiveness and safety of rituximab in multiple sclerosis: an observational study from southern Switzerland. PLoS One 13:e0197415. https://doi.org/10.1371/journal.pone.0197415
Ineichen BV, Moridi T, Granberg T, Piehl F (2019) Rituximab treatment for multiple sclerosis. Mult Scler J 135245851985860:137–152. https://doi.org/10.1177/1352458519858604
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24:96–120. https://doi.org/10.1177/1352458517751049
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 33:1444–1452. https://doi.org/10.1212/WNL.33.11.1444
Kappos L, Butzkueven H, Wiendl H, Spelman T, Pellegrini F, Chen Y, Dong Q, Koendgen H, Belachew S, Trojano M, On Behalf of the Tysabri® Observational Program (TOP) Investigators (2018) Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler J 24:963–973. https://doi.org/10.1177/1352458517709619
NIH/NCI (2017) Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. In: Protocol
Giovannoni G, Tomic D, Bright JR, Havrdová E (2017) “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 23:1179–1187. https://doi.org/10.1177/1352458517703193
Cree B, Lamb S, Chin A, Bonovich D, Islar J, Waubant E, Genain C (2004) Tolerability and effects of rituximab (anti-CD20 antibody) in neuromyelitis optica (NMO) and rapidly worsening multiple sclerosis (MS). Neurology 62:06.90
Salzer J, Svenningsson R, Alping P, Novakova L, Björck A, Fink K, Islam-Jakobsson P, Malmeström C, Axelsson M, Vågberg M, Sundström P, Lycke J, Piehl F, Svenningsson A (2016) Rituximab in multiple sclerosis a retrospective observational study on safety and efficacy. Neurology 87:2074–2081
Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, Piehl F (2018) Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 75:320–327. https://doi.org/10.1001/jamaneurol.2017.4011
Berntsson SG, Kristoffersson A, Boström I, Feresiadou A, Burman J, Landtblom AM (2018) Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden—outlier or predecessor? Acta Neurol Scand 138:327–331. https://doi.org/10.1111/ane.12963
Piccio L, Naismith RT, Trinkaus K, Klein RS, Parks BJ, Lyons JA, Cross AH (2010) Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol 67:707–714. https://doi.org/10.1001/archneurol.2010.99
Hawker K, O’Connor P, Freedman MS et al (2009) Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 66:460–471. https://doi.org/10.1002/ana.21867
Naegelin Y, Naegelin P, Von Felten S et al (2019) Association of rituximab treatment with disability progression among patients with secondary progressive multiple sclerosis. JAMA Neurol 76:274–281. https://doi.org/10.1001/jamaneurol.2018.4239
Yamout BI, El-Ayoubi NK, Nicolas J et al (2018) Safety and efficacy of rituximab in multiple sclerosis: a retrospective observational study. J Immunol Res 2018:1–9. https://doi.org/10.1155/2018/9084759
D’Amico E, Zanghì A, Gastaldi M et al (2019) Placing CD20-targeted B cell depletion in multiple sclerosis therapeutic scenario: present and future perspectives. Autoimmun Rev 18:665–672. https://doi.org/10.1016/j.autrev.2019.05.003
Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, Hillert J, Langer-Gould A, Lycke J, Nilsson P, Salzer J, Svenningsson A, Vrethem M, Olsson T, Piehl F, Frisell T (2020) Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol 87:688–699. https://doi.org/10.1002/ana.25701
Valentino P, Marnetto F, Granieri L, Capobianco M, Bertolotto A (2017) Aquaporin-4 antibody titration in NMO patients treated with rituximab: a retrospective study. Neurol Neuroimmunol Neuroinflamm 4:e317. https://doi.org/10.1212/NXI.0000000000000317
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, OPERA I and OPERA II Clinical Investigators (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 376:221–234. https://doi.org/10.1056/NEJMoa1601277
Paul F, Cartron G (2019) Infusion-related reactions to rituximab: frequency, mechanisms and predictors. Expert Rev Clin Immunol 15:383–389
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MP Amato received research grants and honoraria as a speaker and member of advisory boards from Bayer, Biogen, Merck, Novartis, Sanofi Genzyme, Teva, Almirall, and Roche. E Portaccio served as scientific advisory board for Biogen Idec and Merck Serono; received honoraria for speaking and funding for traveling from Biogen, Genzyme, Novartis, Merck, and Teva; and received research support from Merck Serono. L. Pastò and L. Razzolini received editorial grant from Teva, Almirall, Genzyme, and Merck. All other authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the institutional review board of the University of Florence, Italy, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Informed consent was obtained from all patients.
Consent for publication
The authors declare that neither the article nor portions of it have been previously published elsewhere, that the manuscript is not under consideration for publication in another journal, and will not be submitted elsewhere until the Neurol Sci editorial process is completed. All authors consent for the publication of the manuscript in Neurol Sci, should the article be accepted by the Editor-in-Chief upon completion of the refereeing process.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellinvia, A., Prestipino, E., Portaccio, E. et al. Experience with rituximab therapy in a real-life sample of multiple sclerosis patients. Neurol Sci 41, 2939–2945 (2020). https://doi.org/10.1007/s10072-020-04434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04434-1