Abstract
Introduction
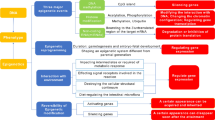
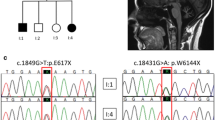
Biallelic mutations in STUB1, which encodes the E3 ubiquitin ligase CHIP, were originally described in association with SCAR16, a rare autosomal recessive spinocerebellar ataxia, so far reported in 16 kindreds. In the last 2 years, a new form of spinocerebellar ataxia (SCA48), associated with heterozygous mutations in the same gene, has been described in 12 kindreds with autosomal dominant inheritance.
Methods
We reviewed molecular and clinical findings of both SCAR16 and SCA48 described patients.
Results and conclusion
SCAR16 is characterized by early onset spastic ataxia and a wide disease spectrum, including cognitive dysfunction, hyperkinetic disorders, epilepsy, peripheral neuropathy, and hypogonadism. SCA48 is an adult-onset syndrome characterized by ataxia and cognitive-psychiatric features, variably associated with chorea, parkinsonism, dystonia, and urinary symptoms. SCA48, the last dominant ataxia to be described, could emerge as the most frequent among the SCAs due to conventional mutations. The overlap of several clinical signs between SCAR16 and SCA48 indicates the presence of a continuous clinical spectrum among recessively and dominantly inherited mutations of STUB1. Different kinds of mutations, scattered over the three gene domains, have been found in both disorders. Their pathogenesis and the relationship between SCA48 and SCAR16 remain to be clarified.


Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its supplementary materials.
References
Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C (1999) Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol 19:4535–4545. https://doi.org/10.1128/mcb.19.6.4535
Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C (2001) CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem 276:42938–42944. https://doi.org/10.1074/jbc.M101968200
Heimdal K, Sanchez-Guixé M, Aukrust I, Bollerslev J, Bruland O, Jablonski GE, Erichsen AK, Gude E, Koht JA, Erdal S, Fiskerstrand T, Haukanes BI, Boman H, Bjørkhaug L, Tallaksen CM, Knappskog PM, Johansson S (2014) STUB1 mutations in autosomal recessive ataxias - evidence for mutation-specific clinical heterogeneity. Orphanet J Rare Dis 9:146. https://doi.org/10.1186/s13023-014-0146-0
Nikolay R, Wiederkehr T, Rist W, Kramer G, Mayer MP, Bukau B (2004) Dimerization of the human E3 ligase CHIP via a coiled-coil domain is essential for its activity. J Biol Chem 279:2673–2678. https://doi.org/10.1074/jbc.M311112200
Cao Z, Li G, Shao Q, Yang G, Zheng L, Zhang T, Zhao Y (2016) CHIP: a new modulator of human malignant disorders. Oncotarget 7:29864–29874. https://doi.org/10.18632/oncotarget.8219
Shi Y, Wang J, Li JD, Ren H, Guan W, He M, Yan W, Zhou Y, Hu Z, Zhang J, Xiao J, Su Z, Dai M, Wang J, Jiang H, Guo J, Zhou Y, Zhang F, Li N, Du J, Xu Q, Hu Y, Pan Q, Shen L, Wang G, Xia K, Zhang Z, Tang B (2013) Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS One 8:e81884. https://doi.org/10.1371/journal.pone.0081884
Shi CH, Schisler JC, Rubel CE, Tan S, Song B, McDonough H, Xu L, Portbury AL, Mao CY, True C, Wang RH, Wang QZ, Sun SL, Seminara SB, Patterson C, Xu YM (2014) Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum Mol Genet 23:1013–1024. https://doi.org/10.1093/hmg/ddt497
Genis D, Ortega-Cubero S, San Nicolás H, Corral J, Gardenyes J, de Jorge L, López E, Campos B, Lorenzo E, Tonda R, Beltran S, Negre M, Obón M, Beltran B, Fàbregas L, Alemany B, Márquez F, Ramió-Torrentà L, Gich J, Volpini V, Pastor P (2018) Heterozygous STUB1 mutation causes familial ataxia with cognitive affective syndrome (SCA48). Neurology. 91:e1988–e1998. https://doi.org/10.1212/WNL.0000000000006550
De Michele G, Lieto M, Galatolo D, Salvatore E, Cocozza S, Barghigiani M, Tessa A, Baldacci J, Pappatà S, Filla A, De Michele G, Santorelli FM (2019) Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: a report of two Italian families. Parkinsonism Relat Disord 65:91–96. https://doi.org/10.1016/j.parkreldis.2019.05.001
Lieto M, Riso V, Galatolo D, De Michele G, Rossi S, Barghigiani M, Cocozza S, Pontillo G, Trovato R, Saccà F, Salvatore E, Tessa A, Filla A, Santorelli FM, De Michele G, Silvestri G (2019) The complex phenotype of spinocerebellar ataxia type 48 in eight unrelated Italian families. Eur J Neurol 65:91–96. https://doi.org/10.1111/ene.14094
Palvadeau R, Kaya-Güleç ZE, Şimşir G, Vural A, Öztop-Çakmak Ö, Genç G, Aygün MS, Falay O, Başak AN, Ertan S (2019) Cerebellar cognitive-affective syndrome preceding ataxia associated with complex extrapyramidal features in a Turkish SCA48 family. Neurogenetics. 21:51–58. https://doi.org/10.1007/s10048-019-00595-0
Coutelier M, Coarelli G, Monin ML, Konop J, Davoine CS, Tesson C, Valter R, Anheim M, Behin A, Castelnovo G, Charles P, David A, Ewenczyk C, Fradin M, Goizet C, Hannequin D, Labauge P, Riant F, Sarda P, Sznajer Y, Tison F, Ullmann U, Van Maldergem L, Mochel F, Brice A, Stevanin G, Durr A, SPATAX network (2017) A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain. 140:1579–1594. https://doi.org/10.1093/brain/awx081
Sun M, Johnson AK, Nelakuditi V, Guidugli L, Fischer D, Arndt K, Ma L, Sandford E, Shakkottai V, Boycott K, Chardon JW, Li Z, Del Gaudio D, Burmeister M, Gomez CM, Waggoner DJ, Das S (2019) Targeted exome analysis identifies the genetic basis of disease in over 50% of patients with a wide range of ataxia-related phenotypes. Genet Med 21:195–206. https://doi.org/10.1038/s41436-018-0007-7
Bettencourt C, de Yébenes JG, López-Sendón JL, Shomroni O, Zhang X, Qian SB, Bakker IM, Heetveld S, Ros R, Quintáns B, Sobrido MJ, Bevova MR, Jain S, Bugiani M, Heutink P, Rizzu P (2015) Clinical and neuropathological features of spastic ataxia in a Spanish family with novel compound heterozygous mutations in STUB1. Cerebellum. 14:378–381. https://doi.org/10.1007/s12311-014-0643-7
Gazulla J, Izquierdo-Alvarez S, Sierra-Martínez E, Marta-Moreno ME, Alvarez S (2018) Inaugural cognitive decline, late disease onset and novel STUB1 variants in SCAR16. Neurol Sci 39:2231–2233. https://doi.org/10.1007/s10072-018-3545-5
Durr A (2010) Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 9:885–894. https://doi.org/10.1016/S1474-4422(10)70183-6
Einfeld SL, Piccinin AM, Mackinnon A, Hofer SM, Taffe J, Gray KM, Bontempo DE, Hoffman LR, Parmenter T, Tonge BJ (2006) Psychopathology in young people with intellectual disability. JAMA. 296:1981–1989. https://doi.org/10.1001/jama.296.16.1981
Theodoratos O, McPherson L, Franklin C, Tonge B, Einfeld S, Lennox N, Ware RS (2017) Psychopathology of adolescents with an intellectual disability who present to general hospital services. Australas Psychiatry 25:481–485. https://doi.org/10.1177/1039856217706820
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain. 121:561–579. https://doi.org/10.1093/brain/121.4.561
Lindsay E, Storey E (2017) Cognitive changes in the spinocerebellar ataxias due to expanded polyglutamine tracts: a survey of the literature. Brain Sci 7:83. https://doi.org/10.3390/brainsci7070083
Rossi M, Perez-Lloret S, Cerquetti D, Merello M (2014) Movement disorders in autosomal dominant cerebellar ataxias: a systematic review. Mov Disord Clin Pract 1:154–160. https://doi.org/10.1002/mdc3.12042
Nakamura K, Jeong SY, Uchihara T, Anno M, Nagashima K, Nagashima T, Ikeda S, Tsuji S, Kanazawa I (2001) SCA17, a novel autosomal dominant cerebellar ataxia caused by an expanded polyglutamine in TATA-binding protein. Hum Mol Genet 10:1441–1448. https://doi.org/10.1093/hmg/10.14.1441
De Michele G, Maltecca F, Carella M, Volpe G, Orio M, De Falco A, Gombia S, Servadio A, Casari G, Filla A, Bruni A (2003) Dementia, ataxia, extrapyramidal features, and epilepsy: phenotype spectrum in two Italian families with spinocerebellar ataxia type 17. Neurol Sci 24:166–167. https://doi.org/10.1007/s10072-003-0112-4
Stevanin G, Brice A (2008) Spinocerebellar ataxia 17 (SCA17) and Huntington's disease-like 4 (HDL4). Cerebellum. 7:170–178. https://doi.org/10.1007/s12311-008-0016-1
Monin ML, Tezenas du Montcel S, Marelli C, Cazeneuve C, Charles P, Tallaksen C, Forlani S, Stevanin G, Brice A, Durr A (2015) Survival and severity in dominant cerebellar ataxias. Ann Clin Transl Neurol 2:202–207. https://doi.org/10.1002/acn3.156
Rosser MF, Washburn E, Muchowski PJ, Patterson C, Cyr DM (2007) Chaperone functions of the E3 ubiquitin ligase CHIP. J Biol Chem 282:22267–22277. https://doi.org/10.1074/jbc.M700513200
Ronnebaum SM, Patterson C, Schisler JC (2014) Emerging evidence of coding mutations in the ubiquitin-proteasome system associated with cerebellar ataxias. Hum Genome 1:14018. https://doi.org/10.1038/hgv.2014.18
Shin Y, Klucken J, Patterson C, Hyman BT, McLean PJ (2005) The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J Biol Chem 280:23727–23734. https://doi.org/10.1074/jbc.M503326200
Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C (2006) CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 440:551–555. https://doi.org/10.1038/nature04600
Miller VM, Nelson RF, Gouvion CM, Williams A, Rodriguez-Lebron E, Harper SQ, Davidson BL, Rebagliati MR, Paulson HL (2005) CHIP suppresses polyglutamine aggregation and toxicity in vitro and in vivo. J Neurosci 25:9152–9161. https://doi.org/10.1523/JNEUROSCI.3001-05.2005
Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, Takahashi R, Murata S, Tanaka K, Takashima A (2005) In vivo evidence of CHIP up-regulation attenuating tau aggregation. J Neurochem 94:1254–1263. https://doi.org/10.1111/j.1471-4159.2005.03272.x
Min JN, Whaley RA, Sharpless NE, Lockyer P, Portbury AL, Patterson C (2008) CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol Cell Biol 28:4018–4025. https://doi.org/10.1128/MCB.00296-08
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):10–17. https://doi.org/10.1016/j.ejmech.2016.07.054
Williams AJ, Paulson HL (2008) Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci 31:521–528. https://doi.org/10.1016/j.tins.2008.07.004
Synofzik M, Puccio H, Mochel F, Schöls L (2019) Autosomal recessive cerebellar ataxias: paving the way toward targeted molecular therapies. Neuron. 101:560–583. https://doi.org/10.1016/j.neuron.2019.01.049
Margolin DH, Kousi M, Chan YM, Lim ET, Schmahmann JD, Hadjivassiliou M, Hall JE, Adam I, Dwyer A, Plummer L, Aldrin SV, O'Rourke J, Kirby A, Lage K, Milunsky A, Milunsky JM, Chan J, Hedley-Whyte ET, Daly MJ, Katsanis N, Seminara SB (2013) Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N Engl J Med 368:1992–2003. https://doi.org/10.1056/NEJMoa1215993
Sawyer SL, Schwartzentruber J, Beaulieu CL, Dyment D, Smith A, Warman Chardon J, Yoon G, Rouleau GA, Suchowersky O, Siu V, Murphy L, Hegele RA, Marshall CR; FORGE Canada Consortium, Bulman DE, Majewski J, Tarnopolsky M, Boycott KM (2014) Exome sequencing as a diagnostic tool for pediatric-onset ataxia. Hum Mutat 35:45–49. https://doi.org/10.1002/humu.22451
Santens P, Van Damme T, Steyaert W, Willaert A, Sablonnière B, De Paepe A, Coucke PJ, Dermaut B (2015) RNF216 mutations as a novel cause of autosomal recessive Huntington-like disorder. Neurology. 84:1760–1766. https://doi.org/10.1212/WNL.0000000000001521
Ganos C, Hersheson J, Adams M, Bhatia KP, Houlden H (2015) The 4H syndrome due to RNF216 mutation. Parkinsonism Relat Disord 21:1122–1123. https://doi.org/10.1016/j.parkreldis.2015.07.012
Alqwaifly M, Bohlega S (2016) Ataxia and hypogonadotropic hypogonadism with Intrafamilial variability caused by RNF216 mutation. Neurol Int 8:6444. https://doi.org/10.4081/ni.2016.6444
Calandra CR, Mocarbel Y, Vishnopolska SA, Toneguzzo V, Oliveri J, Cazado EC, Biagioli G, Turjanksi AG, Marti M (2019) Gordon Holmes Syndrome caused by RNF216 novel mutation in 2 Argentinean siblings. Mov Disord Clin Pract 6:259–262. https://doi.org/10.1002/mdc3.12721
Lieto M, Galatolo D, Roca A, Cocozza S, Pontillo G, Fico T, Pane C, Saccà F, De Michele G, Santorelli FM, Filla A (2019) Overt Hypogonadism may not be a sentinel sign of RING finger protein 216: two novel mutations associated with ataxia, chorea, and fertility. Mov Disord Clin Pract 6:724–726. https://doi.org/10.1002/mdc3.12839
Al-Ramahi I, Lam YC, Chen HK, de Gouyon B, Zhang M, Pérez AM, Branco J, de Haro M, Patterson C, Zoghbi HY, Botas J (2006) CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem 281:26714–26724
Jana NR, Dikshit P, Goswami A, Kotliarova S, Murata S, Tanaka K, Nukina N (2005) Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J Biol Chem 280:11635–11640. https://doi.org/10.1074/jbc.M412042200
Orr HT, Chung MY, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL, McCall AE, Duvick LA, Ranum LP, Zoghbi HY (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4:221–226. https://doi.org/10.1038/ng0793-221
Takiyama Y, Sakoe K, Soutome M, Namekawa M, Ogawa T, Nakano I, Igarashi S, Oyake M, Tanaka H, Tsuji S, Nishizawa M (1997) Single sperm analysis of the CAG repeats in the gene for Machado-Joseph disease (MJD1): evidence for non-Mendelian transmission of the MJD1 gene and for the effect of the intragenic CGG/GGG polymorphism on the intergenerational instability. Hum Mol Genet 6:1063–1068. https://doi.org/10.1093/hmg/6.7.1063
Dickey CA, Patterson C, Dickson D, Petrucelli L (2007) Brain CHIP: removing the culprits in neurodegenerative disease. Trends Mol Med 13:32–38. https://doi.org/10.1016/j.molmed.2006.11.003
McLaughlin B, Buendia MA, Saborido TP, Palubinsky AM, Stankowski JN, Stanwood GD (2012) Haploinsufficiency of the E3 ubiquitin ligase C-terminus of heat shock cognate 70 interacting protein (CHIP) produces specific behavioral impairments. PLoS One 7:e36340. https://doi.org/10.1371/journal.pone.0036340
Lizama BN, Palubinsky AM, Raveendran VA, Moore AM, Federspiel JD, Codreanu SG, Liebler DC, McLaughlin B (2018) Neuronal preconditioning requires the mitophagic activity of C-terminus of HSC70-interacting protein. J Neurosci 38:6825–6840. https://doi.org/10.1523/JNEUROSCI.0699-18.2018
Guo D, Ying Z, Wang H, Chen D, Gao F, Ren H, Wang G (2015) Regulation of autophagic flux by CHIP. Neurosci Bull 31:469–479. https://doi.org/10.1007/s12264-015-1543-7
Rao L, Sha Y, Eissa NT (2017) The E3 ubiquitin ligase STUB1 regulates autophagy and mitochondrial biogenesis by modulating TFEB activity. Mol Cell Oncol 4(6):e1372867. https://doi.org/10.1080/23723556.2017.1372867
Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT (2017) STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J 36:2544–2552. https://doi.org/10.15252/embj.201796699
Wei Q, Sha Y, Bhattacharya A, Abdel Fattah E, Bonilla D, Jyothula SS, Pandit L, Khurana Hershey GK, Eissa NT (2014) Regulation of IL-4 receptor signaling by STUB1 in lung inflammation. Am J Respir Crit Care Med 189:16–29. https://doi.org/10.1164/rccm.201305-0874OC
Matsumura Y, Sakai J, Skach WR (2013) Endoplasmic reticulum protein quality control is determined by cooperative interactions between Hsp/c70 protein and the CHIP E3 ligase. J Biol Chem 288:31069–31079. https://doi.org/10.1074/jbc.M113.479345
Pakdaman Y, Sanchez-Guixé M, Kleppe R, Erdal S, Bustad HJ, Bjørkhaug L, Haugarvoll K, Tzoulis C, Heimdal K, Knappskog PM, Johansson S, Aukrust I (2017) In vitro characterization of six STUB1 variants in spinocerebellar ataxia 16 reveals altered structural properties for the encoded CHIP proteins. Biosci Rep 37. https://doi.org/10.1042/BSR20170251
Kanack AJ, Newsom OJ, Scaglione KM (2018) Most mutations that cause spinocerebellar ataxia autosomal recessive type 16 (SCAR16) destabilize the protein quality-control E3 ligase CHIP. J Biol Chem 293:2735–2743. https://doi.org/10.1074/jbc.RA117.000477
Madrigal SC, McNeil Z, Sanchez-Hodge R, Shi CH, Patterson C, Scaglione KM, Schisler JC (2019) Changes in protein function underlie the disease spectrum in patients with CHIP mutations. J Biol Chem 294:19236–19245. https://doi.org/10.1074/jbc.RA119.011
Chen DH, Latimer C, Yagi M, Ndugga-Kabuye MK, Heigham E, Jayadev S, Meabon JS, Gomez CM, Keene CD, Cook DG, Raskind WH, Bird TD (2020) Heterozygous STUB1 missense variants cause ataxia, cognitive decline, and STUB1 mislocalization. Neurol Genet 6:1–13. https://doi.org/10.1212/NXG.0000000000000397
Mol MO, van Rooij JGJ, Brusse E, Verkerk AJMH, Melhem S, den Dunnen WFA, Rizzu P, Cupidi C, van Swieten JC, Donker Kaat L (2020) Clinical and pathologic phenotype of a large family with heterozygous STUB1 mutation. Neurol Genet 6:e417. https://doi.org/10.1212/NXG.0000000000000417
Synofzik M, Schüle R, Schulze M, Gburek-Augustat J, Schweizer R, Schirmacher A, Krägeloh-Mann I, Gonzalez M, Young P, Züchner S, Schöls L, Bauer P (2014) Phenotype and frequency of STUB1 mutations: next-generation screenings in Caucasian ataxia and spastic paraplegia cohorts. Orphanet J Rare Dis 9:57. https://doi.org/10.1186/1750-1172-9-57
Depondt C, Donatello S, Simonis N, Rai M, van Heurck R, Abramowicz M, D'Hooghe M, Pandolfo M (2014) Autosomal recessive cerebellar ataxia of adult onset due to STUB1 mutations. Neurology. 82:1749–1750. https://doi.org/10.1212/WNL.0000000000000416
Cordoba M, Rodriguez-Quiroga S, Gatto EM, Alurralde A, Kauffman MA (2014) Ataxia plus myoclonus in a 23-year-old patient due to STUB1 mutations. Neurology. 83:287–288. https://doi.org/10.1212/WNL.0000000000000600
Kawarai T, Miyamoto R, Shimatani Y, Orlacchio A, Kaji R (2016) Choreoathetosis, dystonia, and myoclonus in 3 siblings with autosomal recessive spinocerebellar ataxia type 16. JAMA Neurol 73:888–890. https://doi.org/10.1001/jamaneurol.2016.0647
Hayer SN, Deconinck T, Bender B, Smets K, Züchner S, Reich S, Schöls L, Schüle R, De Jonghe P, Baets J, Synofzik M (2017) STUB1/CHIP mutations cause Gordon Holmes syndrome as part of a widespread multisystemic neurodegeneration: evidence from four novel mutations. Orphanet J Rare Dis 12:31. https://doi.org/10.1186/s13023-017-0580-x
Turkgenc B, Sanlidag B, Eker A, Giray A, Kutuk O, Yakicier C, Tolun A, Temel SG (2018) STUB1 polyadenylation signal variant AACAAA does not affect polyadenylation but decreases STUB1 translation causing SCAR16. Hum Mutat 39:1344–1348. https://doi.org/10.1002/humu.23601
Acknowledgements
The study was partially supported by a grant from the Italian Association for Ataxic Syndromes (AISA) to A.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no financial interests in this manuscript.
Ethical approval
None.
Human and animal rights and informed consent
This article is a review of the literature; therefore, it did not involve human participants and did not need informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanna De Michele and Daniele Galatolo shared the first position.
Appendix
Appendix
Addendum
After submission, two articles have been published, describing three novel SCA48 families, two from the USA [58] and one from the Netherlands [59]. The papers confirm the complex phenotype of the disease and add valuable information on its neuropathology. Both studies describe marked loss of Purkinje cells (PC) with Bergmann gliosis, and both did not find TDP-43 or alpha-synuclein positive inclusions. The Dutch study also revealed severe neuronal loss in the mesencephalon and medulla oblongata. Aberrant STUB1 localization in the distal PC dendritic arbors [58] and ubiquitin/p62-positive neuronal inclusions in the cerebellum, neocortex, and brainstem [59] have been also described.
Rights and permissions
About this article
Cite this article
De Michele, G., Galatolo, D., Barghigiani, M. et al. Spinocerebellar ataxia type 48: last but not least. Neurol Sci 41, 2423–2432 (2020). https://doi.org/10.1007/s10072-020-04408-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04408-3