Abstract
Biallelic mutations of the alsin Rho guanine nucleotide exchange factor (ALS2) gene cause a group of overlapping autosomal recessive neurodegenerative disorders including infantile-onset ascending hereditary spastic paralysis (IAHSP), juvenile primary lateral sclerosis (JPLS), and juvenile amyotrophic lateral sclerosis (JALS/ALS2), caused by retrograde degeneration of the upper motor neurons of the pyramidal tracts. Here, we describe 11 individuals with IAHSP, aged 2–48 years, with IAHSP from three unrelated consanguineous Iranian families carrying the homozygous c.1640+1G>A founder mutation in ALS2. Three affected siblings from one family exhibit generalized dystonia which has not been previously described in families with IAHSP and has only been reported in three unrelated consanguineous families with JALS/ALS2. We report the oldest individuals with IAHSP to date and provide evidence that these patients survive well into their late 40s with preserved cognition and normal eye movements. Our study delineates the phenotypic spectrum of IAHSP and ALS2-related disorders and provides valuable insights into the natural disease course.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retrograde degeneration of the upper motor neurons (UMN) of the pyramidal tracts due to biallelic pathogenic ALS2 (OMIM * 606352) variants has been associated with a clinical continuum ranging from infantile-onset ascending hereditary spastic paralysis (IAHSP, OMIM # 607225) to juvenile forms without lower motor neuron (LMN) involvement (juvenile primary lateral sclerosis (JPLS, OMIM # 606353)), and juvenile forms with LMN involvement (autosomal recessive juvenile amyotrophic lateral sclerosis (JALS/ALS2, OMIM # 205100)) [1,2,3,4].
The ALS2 (alsin Rho guanine nucleotide exchange factor) gene, also known as KIAA1563, is located at 2q33.1 and encodes at least two isoforms of the alsin protein, a long form with 1657 amino acids and a short form with 396 amino acids. Both forms are ubiquitously expressed in various tissues, including the brain and spinal cord [1, 2]. Alsin is a member of the guanine nucleotide exchange factors (GEFs) and can activate Rho, Rac1, and Rab5 GTPases. The protein contains three main (GEF) domains: the N-terminal regulator of chromatin condensation (RCC1)-like domain, the central Dbl homology and pleckstrin homology (DH/PH) domain, and the C-terminal vacuolar protein sorting 9 (VPS9) domain [1,2,3, 5,6,7,8,9]. Alsin plays a role in endosomal and mitochondrial trafficking as well as cytoskeleton maintenance and endocytosis [5,6,7,8,9].
Here, we report a novel homozygous splice site ALS2 pathogenic variant, identified using exome sequencing, in 11 individuals with IAHSP from three unrelated consanguineous Iranian families.
Materials and methods
This study was approved by the local ethics committee/Institutional Review Board at Ahvaz Jundishapur University of Medical Sciences, and informed consent was obtained from the three participating families. Members of these families were recruited through the Narges and Noor Medical Genetic Diagnostic Laboratories in Ahvaz, Iran.
Exome sequencing was performed on genomic DNA extracted from blood from the proband of each family (IV:1 in family 1, III:6 in family 2, and IV:1 in family 3). Exome library preparation, sequencing, bioinformatics, and data analysis were performed as previously described [10]. Based on the assumption that the disease follows an autosomal recessive inheritance pattern as well as the presence of consanguinity in these families, homozygous potentially deleterious variants residing within regions of homozygosity (> 2 Mb) were prioritized. These variants were screened through publically available population databases and our in-house database generated for variant frequency in the Iranian population. Synonymous variants, intronic variants (> 5 bp from exon boundaries), and common variants (minor allele frequency > 0.001%) were excluded. Validation and segregation analysis were performed using Sanger sequencing.
Results
Genomic analyses
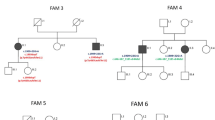
Exome sequencing revealed a novel homozygous ALS2 canonical splice site variant, NM_020919.3: c.1640+1G>A, located in a large homozygous identical-by-descent (IBD) region and co-segregating with IAHSP in the affected siblings, unaffected parents (heterozygous carriers), and healthy siblings (heterozygous carriers and homozygous wild type) in the three families (Fig. 1). The variant is not present in ExAC (Exome Aggregation Consortium, http://exac.broadinstitute.org; accessed February 2018), gnomAD (genome Aggregation Database, http://gnomad.broadinstitute.org; accessed February 2018), GME Variome (Greater Middle East Variome Project, http://igm.ucsd.edu/gme; accessed February 2018), or Iranome (http://iranome.com; accessed February 2018) and was absent from ~ 2000 Iranian control alleles. The variant is present on a common haplotype and represents a founder mutation. The clinical features of all 11 affected individuals are summarized in Table 1.
Pedigrees of the described families. a Pedigree of family 1. b Pedigree of family 2. c Pedigree of family 3. An arrow indicates the proband in each family. d Electropherograms of different individuals in the three Iranian families showing affected members are homozygous for the c.1640+1G>A variant (top), unaffected carriers are heterozygous for the variant (middle), and unaffected non-carriers are homozygous for the wild type allele (bottom)
Family 1
Family 1 consists of two affected and one unaffected sibling born to healthy consanguineous Iranian parents of Arab descent (Table 1; Fig. 1a). The two affected siblings had no history of prenatal, perinatal, or neonatal complications. They sat independently at approximately 6 months old and spoke at the age of 1.5 years. However, they developed mild developmental delay in the second year. They were only able to walk with assistance at 1.5 years and never attained independent walking. Both siblings demonstrated regression with dysarthria and progression to spastic tetraparesis. They have normal cognition, hearing, and vision. They do not have dysphagia, abnormal eye movements, or sensory abnormalities.
Patient IV:1, the proband in family 1, is a 5-year-old male. He developed significant ankle contractures and became wheelchair bound at 4 years old after losing the ability to walk with support despite Achilles tendon release. On examination, he had dysarthria, mild atrophy of the distal limb muscles, weakness of the shoulder girdle muscles, spastic tetraparesis, hyperreflexia, sustained clonus, and bilateral extensor plantar responses. His metabolic testing and brain MRI were normal at the age of 3 years. Results of his electromyography were suggestive of UMN disease.
Patient IV:2 is a 2-year-old female who has a similar phenotype to her older brother IV:1, but she is not restricted to a wheelchair.
Family 2
Family 2 consists of three affected sisters with IAHSP and generalized dystonia as well as six unaffected siblings born to healthy consanguineous Iranian parents of Arab descent (Table 1; Fig. 1b). The three affected females presented with similar clinical findings but with variable age of onset and disease progression. They all have normal cognition, eye movements, bowel and bladder continence, and motor coordination. Motor and sensory nerve conduction studies (NCS) were normal.
The proband in family 2, patient III:6, is a 17-year-old female with a history of normal motor and speech development. She sat and walked unsupported at 6 months and 1 year old respectively. However, her development slowed, and she began to regress during the second year of life. Ascending spastic paresis presented with unilateral leg claudication at the age of 1.5 years. She progressed to spastic tetraparesis and eventually became wheelchair bound at 6 years old. She developed dysarthria at 7, which progressed to anarthria at the age of 8. She also developed other bulbar and pseudobulbar signs as well as opisthotonus at 8 years old.
Patients III:4 and III:8, a 26- and 13-year-old female respectively, unlike their sister (proband III:6), had a history of early onset developmental delay and regression. They never achieved the ability to sit or walk independently, and while they made some vocalizations, they never developed speech. They developed ascending spastic paraparesis during the first year of life. Their condition progressed to spastic tetraparesis and they eventually became wheelchair bound at the age of 6 years. Other bulbar and pseudobulbar signs as well as opisthotonus were present from the age of 7 years for III:4 and before the age of 2 years for III:8. The youngest sister, III:8, had a normal brain MRI.
Family 3
Family 3 consists of seven affected individuals (six with genetic testing) from a large consanguineous Iranian family of Arab descent (Table 1; Fig. 1c). All the patients presented with developmental delay and regression as well as progression to spastic tetraparesis.
Patient IV:1, the proband in family 3, is a 7-year-old female and the first child of her healthy consanguineous Arab parents. She sat unsupported at the age of 9 months. She was late to crawl and was only able to stand with support after starting occupational therapy at 18 months old. She developed ankle contractures and never attained the ability to walk independently. She developed mild dysphagia and dysarthria at the age of 6 years. When examined at 7 years old, anarthria, pseudobulbar palsy, elbow flexion contractures, and mild atrophy of the distal limb muscles were evident. She had mild facial muscle weakness, shoulder girdle muscle weakness, spastic tetraparesis, hyperreflexia, sustained clonus, and bilateral extensor plantar responses. She demonstrated assisted toe walking with a spastic gait. Cognition, eye movements, and cerebellar examination were normal. There were no sensory, visual, or hearing deficits. Her neurometabolic workup and electromyography at the age of 2 years were normal. Her brain MRI was also normal.
Patients III:7–10 are four affected siblings (2 males and 2 females) aged between 42 and 48 years. They sat unsupported at 9 months old. Global developmental delay was recognized during the second year of life. They developed dysarthria and progressive spastic paraparesis never achieving the ability to stand or walk unsupported. Their clinical course deteriorated rapidly with upper limb, bulbar, and pseudobulbar involvement. They developed anarthria, severe dysphagia, trismus (only two of the older siblings), and incontinence, becoming bedbound at approximately 12 years old. On their last examination, they were cachectic with evidence of profound muscle atrophy of the distal limbs and flexion contractures of the knees and ankles. They had facial muscle weakness, spastic tetraparesis, hyperreflexia, sustained clonus, bilateral extensor plantar responses, and mild scoliosis. Their cognition and ocular movements were normal. Brain MRI of the oldest sibling at the age of 47 years showed mild cerebral atrophy.
Patient III:18 is a 38-year-old female who has a similar phenotype to her affected relatives (four siblings, III:7–10) except that she had an earlier onset of developmental delay and regression. Unlike her affected relatives, she never achieved the ability to sit independently and spoke her first word at 3 years old. She only used single words and developed dysarthria which progressed to anarthria at the age of 5 years. Videos of the patients from these three families can be made available on request.
Discussion
Here, we present phenotypic data for 11 affected individuals with IAHSP from three independent Iranian families, all of whom share an identical novel ALS2 canonical splice site mutation (Table 1). Mutations in the ALS2 gene have been associated with a phenotypic spectrum ranging from infantile forms with both UMN and LMN involvement (IAHSP) to juvenile forms with (JALS/ALS2) or without (JPLS) LMN involvement [1,2,3,4, 11, 12]. To date, approximately 50 ALS2 pathogenic variants have been reported in individuals with ALS2-related disorders and include missense, nonsense, frameshift, and splice site variants that are thought to result in loss of function [12]. At least 22 of the reported ALS2 pathogenic variants have been associated with IAHSP and have been described in a total of 42 individuals. The majority of reported cases originate from the Middle East and Mediterranean countries [2,3,4, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. Table 2 provides a summary of the mutations and clinical features of the previously reported IAHSP cases.
IAHSP is characterized by the onset of spastic paraparesis within the first 2 years of life. Some children never walk independently or speak, while others are slow to walk and/or speak and then regress and lose the ability to walk independently and/or speak. The disease progresses with upper limb, bulbar, and pseudobulbar involvement to severe spastic tetraparesis, anarthria, dysphagia, and slow eye movements during the first decade of life. Wheelchair dependence occurs in the second decade of life. Incontinence and feeding via a gastrostomy have been described in some individuals in the advanced stages of the disease [17]. Scoliosis, although reported, is uncommon. Overall, cognitive function is preserved in IAHSP, and the condition is compatible with long-term survival. Muscle biopsy and nerve conduction velocities are normal, and electromyography reveals no sign of denervation. However, motor evoked potentials show signs of severe dysfunction of the corticospinal tracts, consistent with degeneration of UMN. Somatosensory evoked potentials are normal in the early stages and abnormal in later stages of the disease. Brain MRI is normal in children, but older patients had cortical atrophy (predominantly in the motor areas) and T2-weighted bilateral hyperintensities in the corticospinal pathways of the posterior arms of the internal capsule and brainstem. In addition, T2 and FLAIR periventricular hyperintensities and cervical cord atrophy that are frequently seen in other hereditary spastic paraplegias (HSPs) are commonly identified [4, 12].
The novel phenotype of JALS/ALS2 associated with generalized dystonia has previously been reported in two unrelated consanguineous families of Bangladeshi and Turkish descent, with homozygous loss of function variants in ALS2 [26]. Shortly thereafter, the same phenotype was described in a large consanguineous Pakistani family with a homozygous splice site variant in ALS2 [27]. Other novel clinical findings in the Bangladeshi family described in the first paper [26] include microcephaly and cerebellar signs, but the authors noted that it is unclear whether these features are caused by the ALS2 pathogenic variant.
Our study provides one of the largest reported series and includes the oldest reported individuals with IAHSP surviving into their late 40s with preserved cognitive function and normal eye movements. To our knowledge, these are the first Iranian cases affected by IAHSP and ALS2-related disorders. Additionally, here, we report on the first family in the literature with IAHSP and generalized dystonia (family 2). Despite having the identical homozygous ALS2 pathogenic variant, these families demonstrate intra- and interfamilial phenotypic variability, suggesting that environmental factors, modifier genes, and/or epigenetic factors may play a role in this condition.
In conclusion, we identified a novel ALS2 pathogenic founder variant in Iran that further adds to the allelic heterogeneity of IAHSP. The family with generalized dystonia expands the phenotypic variability of IAHSP and ALS2-related disorders. These findings also demonstrate the overlapping nature of these allelic disorders. Future clinical and functional studies are needed to better delineate genotype-phenotype correlations.
References
Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, Hung WY, Ouahchi K, Yan J, Azim AC, Cole N, Gascon G, Yagmour A, Ben-Hamida M, Pericak-Vance M, Hentati F, Siddique T (2001) The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet 29(2):160–165
Hadano S, Hand CK, Osuga H, Yanagisawa Y, Otomo A, Devon RS, Miyamoto N, Showguchi-Miyata J, Okada Y, Singaraja R, Figlewicz DA, Kwiatkowski T, Hosler BA, Sagie T, Skaug J, Nasir J, Brown RH, Scherer SW, Rouleau GA, Hayden MR, Ikeda JE (2001) A gene encoding a putative GTPase regulator is mutated in familial amyotrophic lateral sclerosis 2. Nat Genet 29:166–173
Eymard-Pierre E, Lesca G, Dollet S, Santorelli FM, di Capua M, Bertini E, Boespflug-Tanguy O (2002) Infantile-onset ascending hereditary spastic paralysis is associated with mutations in the alsin gene. Am J Hum Genet 71(3):518–527
Lesca G, Eymard-Pierre E, Santorelli FM, Cusmai R, di Capua M, Valente EM, Attia-Sobol J, Plauchu H, Leuzzi V, Ponzone A, Boespflug-Tanguy O, Bertini E (2003) Infantile ascending hereditary spastic paralysis (IAHSP): clinical features in 11 families. Neurology 60(4):674–682
Otomo A, Hadano S, Okada T, Mizumura H, Kunita R, Nishijima H, Showguchi-Miyata J, Yanagisawa Y, Kohiki E, Suga E, Yasuda M, Osuga H, Nishimoto T, Narumiya S, Ikeda JE (2003) ALS2, a novel guanine nucleotide exchange factor for the small GTPase Rab5, is implicated in endosomal dynamics. Hum Mol Genet 12(14):1671–1687
Topp JD, Gray NW, Gerard RD, Horazdovsky BF (2004) Alsin is a Rab5 and Rac1 guanine nucleotide exchange factor. J Biol Chem 279:24612–24623
Kunita R, Otomo A, Mizumura H, Suzuki K, Showguchi-Miyata J, Yanagisawa Y, Hadano S, Ikeda JE (2004) Homo-oligomerization of ALS2 through its unique carboxyl-terminal regions is essential for the ALS2-associated Rab5 guanine nucleotide exchange activity and its regulatory function on endosome trafficking. J Biol Chem 279(37):38626–38635
Kunita R, Otomo A, Mizumura H, Suzuki-Utsunomiya K, Hadano S, Ikeda JE (2007) The Rab5 activator ALS2/alsin acts as a novel Rac1 effector through Rac1-activated endocytosis. J Biol Chem 282(22):16599–16611
Hadano S, Kunita R, Otomo A, Suzuki-Utsunomiya K, Ikeda JE (2007) Molecular and cellular function of ALS2/alsin: implication of membrane dynamics in neuronal development and degeneration. Neurochem Int 51(2–4):74–84
Dilaver N, Mazaheri N, Maroofian R, Zeighami J, Seifi T, Zamani M, Sedaghat A, Shariati GR, Galehdari H (2017) Novel homozygous missense mutation in RYR1 leads to severe congenital ptosis, ophthalmoplegia, and scoliosis in the absence of myopathy. Mol Syndromol 9(1):25–29
Wakil SM, Ramzan K, Abuthuraya R, Hagos S, al-Dossari H, al-Omar R, Murad H, Chedrawi A, al-Hassnan ZN, Finsterer J, Bohlega S (2014) Infantile-onset ascending hereditary spastic paraplegia with bulbar involvement due to the novel ALS2 mutation c.2761C4T. Gene 536(1):217–220
Orrell RW ALS2-related disorders. In: Adam MP et al (eds) GeneReviews® [Internet]. University of Washington, Seattle (WA) 1993–2017. 2005 Oct 21 [updated 2016 Jan 28]
Lerman-Sagie T, Filiano J, Warwick Smith D, Korson M (1996) Infantile onset of hereditary ascending spastic paralysis with bulbar involvement. J Child Neurol 11(1):54–57
Gros-Louis F, Meijer IA, Hand CK, Dubé MP, MacGregor DL, Seni MH, Devon RS, Hayden MR, Andermann F, Andermann E, Rouleau GA (2003) An ALS2 gene mutation causes hereditary spastic paraplegia in a Pakistani kindred. Ann Neurol 53(1):144–145
Devon RS, Helm JR, Rouleau GA, Leitner Y, Lerman-Sagie T, Lev D, Hayden MR (2003) The first nonsense mutation in alsin results in a homogeneous phenotype of infantile-onset ascending spastic paralysis with bulbar involvement in two siblings. Clin Genet 64(3):210–215
Eymard-Pierre E, Yamanaka K, Haeussler M, Kress W, Gauthier-Barichard F, Combes P, Cleveland DW, Boespflug-Tanguy O (2006) Novel missense mutation in ALS2 gene results in infantile ascending hereditary spastic paralysis. Ann Neurol 59(6):976–980
Verschuuren-Bemelmans CC, Winter P, Sival DA, Elting JW, Brouwer OF, Müller U (2008) Novel homozygous ALS2 nonsense mutation (p.Gln715X) in sibs with infantile-onset ascending spastic paralysis: the first cases from northwestern Europe. Eur J Hum Genet 16(11):1407–1411
Sztriha L, Panzeri C, Kálmánchey R, Szabó N, Endreffy E, Túri S, Baschirotto C, Bresolin N, Vekerdy Z, Bassi MT (2008) First case of compound heterozygosity in ALS2 gene in infantile-onset ascending spastic paralysis with bulbar involvement. Clin Genet 73(6):591–593
Herzfeld T, Wolf N, Winter P, Hackstein H, Vater D, Müller U (2009) Maternal uniparental heterodisomy with partial isodisomy of a chromosome 2 carrying a splice acceptor site mutation (IVS9-2A>T) in ALS2 causes infantile-onset ascending spastic paralysis (IAHSP). Neurogenetics 10(1):59–64
Racis L, Tessa A, Pugliatti M, Storti E, Agnetti V, Santorelli FM (2014) Infantile-onset ascending hereditary spastic paralysis: a case report and brief literature review. Eur J Paediatr Neurol 18(2):235–239
Flor-de-Lima F et al (2014) Alsin related disorders: literature review and case study with novel mutations. Case Rep Genet 2014:691515
Eker HK et al (2014) A novel homozygous mutation in ALS2 gene in four siblings with infantile onset ascending hereditary spastic paralysis. Eur J Med Genet 57(6):275–278
Xie F, Cen ZD, Xiao JF, Luo W (2015) Novel compound heterozygous ALS2 mutations in two Chinese siblings with infantile ascending hereditary spastic paralysis. Neurol Sci 36(7):1279–1280
Daud S, Kakar N, Goebel I, Hashmi AS, Yaqub T, Nürnberg G, Nürnberg P, Morris-Rosendahl DJ, Wasim M, Volk AE, Kubisch C, Ahmad J, Borck G (2016) Identification of two novel ALS2 mutations in infantile-onset ascending hereditary spastic paraplegia. Amyotroph Lateral Scler Frontotemporal Degener 17(3–4):260–265
Tariq H, Mukhtar S, Naz S (2017) A novel mutation in ALS2 associated with severe and progressive infantile onset of spastic paralysis. J Neurogenet 31(1–2):26–29
Sheerin UM, Schneider SA, Carr L, Deuschl G, Hopfner F, Stamelou M, Wood NW, Bhatia KP (2014) ALS2 mutations: juvenile amyotrophic lateral sclerosis and generalized dystonia. Neurology 82(12):1065–1067
Siddiqi S, Foo JN, Vu A, Azim S, Silver DL, Mansoor A, Tay SKH, Abbasi S, Hashmi AH, Janjua J, Khalid S, Tai ES, Yeo GW, Khor CC (2014) A novel splice-site mutation in ALS2 establishes the diagnosis of juvenile amyotrophic lateral sclerosis in a family with early onset anarthria and generalized dystonias. PLoS One 9(12):e113258
Acknowledgments
We would like to thank the patients and their families for their participation and generous contributions. Additionally, we would also like to thank Lara Dilaver, graphic designer, for editing patients’ videos.
Funding
This study was funded by Rare Disease Foundation. TBH was supported by the German Bundesministerium für Bildung and Forschung (BMBF) through the Juniorverbund in der Systemmedizin “mitOmics” (FKZ 01ZX1405C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the local ethics committee/Institutional Review Board at Ahvaz Jundishapur University of Medical Sciences, and informed consent was obtained from the three participating families.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Helal, M., Mazaheri, N., Shalbafan, B. et al. Clinical presentation and natural history of infantile-onset ascending spastic paralysis from three families with an ALS2 founder variant. Neurol Sci 39, 1917–1925 (2018). https://doi.org/10.1007/s10072-018-3526-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3526-8