Abstract
Patients with Parkinson’s disease (PD) are compromised by poor oral condition due to oropharyngeal bradykinesia, dysphagia, and the side effects of treatment. Intrasalivary gland injections of Botulinum neurotoxin type A (BNT-A) have been known to treat sialorrhea effectively in these patients. However, the decreased amount of saliva reduces self-cleaning ability that deteriorates oral hygiene and increases dental caries. The aim of this study was to determine the changes in the oral microflora and saliva in patients with PD treated for sialorrhea by means of sonography-controlled BNT-A injections into the bilateral parotid and submandibular glands. Altogether, 38 persons participated in the study: 12 PD patients who were injected with BNT-A for treatment of sialorrhea and passed salivary tests before and 1 month after the injections; and 13 PD patients and 13 healthy subjects who were not injected with BNT-A and passed salivary tests once. The condition of oral health was measured by the amount of saliva, salivary flow rate, and salivary composition. A good outcome with a significant decrease in salivary flow rate occurred at 1-month follow-up in the BNT-A-treated group while no significant change was found in salivary composition. BNT-A treatment did not change the Streptococcus mutans levels in saliva but there was statistically significant increase in levels of Lactobacilli. BNT-A injections can effectively treat sialorrhea while considering the change of oral microflora, and the patients should be under dentists’ care more frequently. EudraCT clinical trial number: 2015-000682-30.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the clinical diagnosis of PD is based upon a defined motor syndrome (bradykinesia, rigidity, rest tremor), also, non-motor features are present in most patients in the early stages of the disease already, and often can dominate among clinical manifestations [1,2,3]. As the disease progresses, the burden of non-motor symptoms rises, affecting substantially the quality of life of patients with PD [4, 5]. Some of the non-motor features, such as autonomic and neuropsychiatric disturbances, seem to preferentially affect patients with non-tremor-dominant subtypes of PD [6].
Drooling is not among the most frequent non-motor symptoms with its prevalence ranging from 10 to 84% across different studies and it may affect the quality of life remarkably [7,8,9,10]. It is defined as the inability to control oral secretions, resulting in excessive saliva accumulation in the oropharynx. Usually, the main problem in PD with saliva excess is related to the dysfunctional oral motor control when the glutation process is abnormal due to the weak muscle function [7,8,9, 11]. Based on yet unpublished data of the PD prevalence study in Estonia, excessive drooling was commonly described affecting 47% patients but mostly not as a severe symptom.
Higher odds of the occurrence of drooling have been found among patients with older age, more severe PD, and longer disease duration [8]. Several case-control studies have demonstrated that patients with PD have lower salivary flow [12, 13], but increased excretion velocity to stimulus [7] compared to healthy controls. Drooling may also be associated with oropharyngeal bradykinesia [9], hypomimia, and dysphagia [8, 9]. There is some evidence that levodopa might stimulate salivary flow rate and lead to excessive amount of saliva [12].
Saliva has a purifying and disinfective effect due to its lysosomal content. As the parotid gland produces water, electrolytes, and proteins, saliva is serous. Saliva is also viscous as the submandibular and sublingual glands produce mucopolysaccharides [14]. The physiology of swallowing consists of three phases: voluntary oral, and involuntary pharyngeal and esophageal [10]. Complaints of PD patients depend largely on disturbances occurring in the oropharyngeal phases. Reduced fine motor skills due to the disease may lead to inadequate cleaning of teeth and poor oral condition [15].
Botulinum neurotoxin type A (BNT-A) injections into the salivary glands have been proven to effectively reduce drooling according to several clinical studies [16,17,18]. This treatment method may also affect the oral condition since a change in the amount of saliva is frequently accompanied by changes in salivary pH, oral flora, and saliva characteristics [10]. A decrease in the amount of saliva is thought to be associated with increased incidence of dental caries.
The aim of the present study was to assess the salivary parameters along with the saliva flow in order to improve the knowledge of the oral health management of elderly people and patients with PD and through that evaluate the impact of BNT-A injection therapy on the oral health of PD patients.
Subjects and methods
Thirty-eight study subjects (16 female and 22 male; age range 58–88 years, mean age of 71.1 years) screened at the Tartu University Hospital from April 2015 to January 2016 were enrolled in the prospective, clinical trial. The subjects were selected from the cohort of patients included in the PD epidemiology study at the Department of Neurology and Neurosurgery of Tartu University. Thirteen healthy, age-matched controls (7 female and 6 male) were recruited from the Department of Stomatology.
The patient recruitment was based on screening by the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) questionnaire, Item 2.2 from Part II (Non-Motor Aspects of Experiences of Daily Living) [19]. Demographic and clinical data including age at PD onset, disease duration, clinical motor phenotype, and information on antiparkinsonian treatment were collected for all the PD patients. The clinical motor phenotype of disease was classified on the basis of the presence of leading symptoms: tremor-dominant, akinetic-rigid-dominant, or postural instability and gait disorder-dominant (PIGD). All except one PD patient was clinically thoroughly examined with the use of parts II and III of the MDS-UPDRS and the Hoehn and Yahr Scale [20].
All subjects were divided into three groups: group 1 consisted of 12 PD patients (9 male and 3 female) who suffered from sialorrhea and received BNT-A injections into the salivary glands, group 2 consisted of 13 PD patients without hypersalivation (7 male and 6 female) and who did not receive BNT-A injections, and group 3 consisted of 13 age-matched healthy controls (6 male and 7 female). Group 1 patients passed salivary tests before and 1 month after the injections. Groups 2 and 3 passed salivary tests once.
Inclusion criteria of the study groups were defined as follows. BNT-A injections into salivary glands were used to treat patients suffering from average or severe hypersalivation when logopedical treatment with chewing muscle and orbicularis oris myogymnastics had not been effective in decreasing the saliva flow. PD patients with sialorrhea who scored 1 to 4 based on item 2.2 of the Part II of the MDS-UPDRS were included in group 1. PD patients without sialorrhea whose score of item 2.2 of the MDS-UPDRS was 0 were included in group 2. All the controls in group 3 were healthy volunteers chosen from the similar age group as the PD patients. Patients who received any other sialorrhea treatment were excluded from the study. Another exclusion criterion was the use of any medication during the study that could influence the severity of drooling. One patient from group 1 was excluded from the study because of his refusal of the second follow-up examination.
Intervention
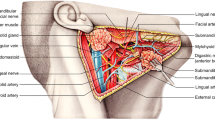
Group 1 was injected with BNT-A (a total of 250 units Dysport) into the salivary glands to treat hypersalivation. The procedure was performed by a maxillofacial surgeon. 27-G needles were placed in the anteroposterior direction into each submandibular and parotid gland under an ultrasound guidance (Fig. 1). Groups 2 and 3 did not receive any treatment influencing the salivary glands. All participants were interviewed about changes in saliva.
To investigate the salivary parameters, saliva from all the study participants was collected in a cup during 5 min; the amount, composition, and microbial state were compared between the groups. The samples were taken before noon, 2 h after the last meal. Patients were instructed not to brush their teeth on the morning before the investigation.
Saliva analysis consisted of the measurement of both resting and stimulated saliva. To measure the amount of stimulated saliva production, the patients chewed a piece of wax for 5 min before the collection of saliva, which accumulated in the oral cavity. Resting saliva was tested by its hydration level, consistency, and pH. Quantity and buffering capacity of stimulated saliva were measured. Salivary levels of the cariogenic bacteria Streptococcus mutans were measured using the Dentocult SM test, and Lactobacilli were measured by using the Dentocult LB test (Orion Diagnostica Co Ltd., Epsom, Finland) [21]. Microbial tests were evaluated by counting colony-forming units (CFU/ml): class 0 = < 103; class 1 = 103–104; class 2 = 104–105; class 3= > 105.
Analysis of saliva composition was performed using the Saliva-Check BUFFER in Vitro Test (GC EUROPE N.V. B-3001 Leuven, Belgium) [22] by the instructions. The change in the amount and composition of saliva during 1 month (before and 1 month after the injection) was measured among patients of group 1 who received BNT-A injections. The results for groups 1, 2, and 3 were compared to assess differences in saliva in PD patients with sialorrhea treated with BNT-A, the PD patients without sialorrhea, and the healthy controls.
Statistical analysis
Data analysis was performed using the IBM SPSS Statistics V20 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were expressed as estimates of percentages, means with standard deviation (SD), and medians with ranges. The Kruskal–Wallis test or ANOVA was used for multiple comparisons. To compare the differences of the variables of interest between the two independent groups, the two-sample t test, the two-proportion z test, and Mann–Whitney test were used as appropriate. Wilcoxon matched pairs signed-ranks test was used for the analysis of non-parametric data, while paired t test was used for parametric data analysis. The Pearson correlation analysis was used to evaluate the associations between variables. The p values < 0.05 were considered statistically significant.
Ethics approval
The study was conducted in accordance with the national and international ethics standards, and was approved by the Research Ethics Committee of the University of Tartu (Protocol No. 221/T-15). Written informed consent was obtained from all participants. As the patients of group 1 received BNT-A injections, the study was approved by the Estonian State Agency of Medicines (Protocol No. 01-09.02.15; EudraCT number: 2015-000682-30).
Results
The mean age for group 1 was 71.3 years (SD 8.5); for group 2, 71.5 years (SD 8.1); and for group 3, 70.6 years (SD 9.0). These differences were statistically insignificant by ANOVA (p = 0.966). The summary of the clinical characteristics of the PD patients from group 1 and group 2 is demonstrated in Table 1. According to the Kruskal–Wallis test and ANOVA, there were no statistically significant differences between the three groups in resting saliva formation (p = 0.372), consistency (p = 0.585), amount of saliva collected during 5 min (p = 0.493), pH (p = 0.635), and buffering capacity (p = 0.183).
By the Pearson correlation analysis, the amount of saliva was larger in patients who were treated with levodopa (p = 0.016), and less in the patients who received MAO-B treatment (p = 0.020). The mean duration of levodopa treatment was significantly longer among patients in group 1 who received BNT-A treatment for sialorrhea, compared to patients in group 2 (Table 1) that indicates to the association between levodopa treatment duration and sialorrhea.
Resting time saliva formation was slower in patients of later disease onset of PD. Resting saliva formation shows the time how quickly a drop of saliva appears from the minor salivary gland of the lower lip. The values before the BNT-A injections were somewhat lower compared to the corresponding values 1 month after the injections (Table 2). The Pearson correlation analysis showed an association between the initial amount of 5-min saliva and the leading symptom (p = 0.016) comparing PD patients in groups 1 and 2. Akinesia-rigidity was the most frequent disease subtype among patients in group 1, and tremor was the most frequent leading PD symptom among patients in group 2 (Table 1).
All patients in group 1 reported saliva thickening 1 month after BNT-A injections. Drooling was very intensive at the baseline; however, after 1 month of treatment, the patients reported a decrease in drooling to the moderate level (p = 0.01). The consistency of saliva did not change significantly according to the Mann–Whitney U test (p = 0.059). However, the amount of the 5-min saliva showed a significant decrease from pre-injection to 1-month post-injection assessment (Table 2). In group 1, comparison of pH values revealed no difference before and 1 month after the injection (p = 1.000), which is an evidence of preserved normal pH. Buffering capacity was higher 1 month after BNT-A treatment compared to the pre-injection value (p = 0.037) (Table 2). It shows that the ability of saliva to maintain the normal oral pH was better after injection than before.
According to the Mann–Whitney U test, there was no significant change in the count of S. mutans values, but Lactobacilli counts rose (Table 2): the Dentocult SM values were similar before, and 1 month after the BNT-A treatment, and the Dentocult LB values increased, being higher 1 month after the injection.
PD patients and their carers were asked to report adverse events after the BNT-A injections but there were no adverse events, and the treatment was generally well tolerated. There were no complaints of swelling or pain in group 1.
Discussion
This study focused on BNT-A treatment for sialorrhea in PD patients, and the aim of the study was to evaluate the impact of BNT-A injection therapy on the saliva properties. Other parameters of the general oral condition were not assessed. There is a number of specific orofacial problems affecting patients’ oral condition even without possible BNT-A treatment complications, but associated with PD, including muscular hypokinesia, rigidity, and malnutrition in a case of decreased liquid intake and eating soft sticky food [15]. Oral implications like xerostomia, bruxism, dry throat, gingivitis, tongue edema, abnormal taste, glossitis, and orthostatic hypotension may result from the adverse effects of PD medications [23, 24]. Patients with PD have more food debris and poorer oral clearance, as well as more dental plaque, caries, and poor periodontal health [25]. This leads to more missing teeth and various denture problems, which have been ascribed to lack of orofacial muscular control, hyposalivation, and compromised manual skillfulness. PD patients report more often oral health-related problems compared to age- and gender-matched control subjects, and the problems increase significantly with increasing UPDRS score for motor impairment [26]. Caries, periodontal disease, and tooth loss may occur due to the inability to perform proper oral hygiene; also, the chewing process is less efficient because of tremor, rigidity, and hypokinesia [9]. Reduced jaw mobility and slowness of jaw movements as well as dysphagia-related food retention are common problems [26]. Patients with PD may be apathetic, depressive, or demented, and thus may not notice their dental problems. Poor oral and periodontal health is a risk factor for general health problems, including cardiovascular diseases, ischemic stroke, diabetes mellitus, atherosclerosis, pulmonary diseases, and rheumatoid arthritis [27, 28].
The standard medication of PD is levodopa. However, the effect of treatment decreases with time, and after 5–10 years, treatment complications may occur [4, 5, 26]. Earlier studies have shown that most patients with PD produce less saliva compared to healthy controls [10, 16, 26]. This can be explained with dopamine deficiency that modulates salivary secretion [15, 29]. Levodopa stimulates the rate of both basal and reflex salivary flow and leads to saliva excess in some cases of PD [12, 30]. The prevalence of drooling in PD patients ranges widely, from 10 to 84% [10, 16]. Our study showed that PD patients who experienced drooling were statistically more often on levodopa treatment supporting the findings by Ou et al. [31].
However, there are little data on the association between drooling and motor subtypes of the disease. Current study revealed that PD patients with tremor-dominant subtype of disease reported drooling less frequently than those with akinetic-rigid and PIGD-dominant subtype of PD. This is consistent with the results by Karakoc et al. showing that bradykinesia scores in MDS-UDPRS Part III were significantly higher in droolers than those in non-droolers [9]. PIGD and akinetic-rigid subtypes of PD have been linked with higher burden of non-motor features; therefore, the management of those patients might be more complex than of those who predominantly experience tremor [6].
Treatment of drooling with BNT-A injections has an effect on the amount and condition of saliva and leads to the changes of oral environment and health. Our results are in line with previous studies showing that BNT-A reduces drooling [18, 31,32,33,34]. There are no data from earlier studies on saliva composition and microbial changes after BNT-A.
Submandibular and parotid glands have been chosen for targets of BNT-A injections to treat sialorrhea because in the resting state, the saliva is mostly produced by the submandibular gland (71%), also by the parotid gland (25%), and less by the sublingual gland (4%) [10] and after stimulation, 63% of saliva is produced by the submandibular gland, 34% by the parotid gland, and 3% by the sublingual gland [14]. Our results on resting saliva formation indicate that the oral cavity remained hydrated and healthy during the treatment with BNT-A. This can be explained by the fact that the salivary flow from the greater salivary glands was affected but not from the minor salivary glands. In our study, no significant alterations in salivary pH were demonstrated after the BNT-A injections. Salivary buffering capacity shows how quickly the oral pH can reach its normal value after a meal. Saliva possesses buffering capacity for neutralizing acids present in the mouth. It can be attributed to several systems such as the phosphate system and the carbonic acid/bicarbonate system. That is why saliva can maintain its usual pH after a meal. Salivary buffers are resistant to changes in pH [14]. Buffering capacity increased during the study period, indicating improvement in salivary defense ability. Saliva’s normal pH is 6.7–7.4 [14]. When the pH of saliva drops below 5.5, demineralization of tooth enamel usually follows. In the acid oral pH state, there is a higher risk for dental caries, and in the alkaline pH condition, a higher risk for the dental calculus [30]. Beside the low pH and buffering capacity values, cariogenic bacterial appearance plays a key role in caries formation and progression. Most important of them are S. mutans and Lactobacilli. Our study showed no change in S. mutans values, but Lactobacilli counts were increased 1 month after the injection. Some earlier studies have reported periodontal disorders and a worsened dental condition in subjects with PD [15, 35]. However, studies of oral and dental health with a focus on the microbial status and salivary health of patients with PD have so far been lacking. It is generally known that the most effective way to prevent progression of periodontal disease is brushing of teeth. Consequently, maintaining the oral condition can be improved through the use of powered toothbrushes or special antimicrobial rinses that minimize oral infections. For PD patients, routine regular cleaning of teeth with powered toothbrushes could be easier to perform, and not affected too much by motor and cognitive dysfunctions including tremor, akinesia, rigidity, and dementia. When recommending mouth rinses, caution should be advised as rinsing may lead to aspiration because of the dysfunction of the oral musculature. Also, the use of fluoridated products and calcium phosphate can reduce the risk of caries and help strengthen the enamel; sodium fluoride may reduce the risk of root caries [23].
Patients with PD have poor oral health depending on the symptoms of the main disease. Although, the results of the study showed no statistically significant changes in salivary composition before and after BNT-A injections, the level of Lactobacilli counts raised after BNT-A injections. The main limitation of the study is the relatively small sample size of the study groups that could be a source of low statistical power. Another limitation is the fact that only limited parameters of saliva including cariogenic microflora were assessed but not the oral health in more details. Future research to evaluate different Lactobacilli subtypes and their influence to the oral health should be done. Also, there is a need for studies containing periodontal pathogens. In parallel with achieved therapeutic effect of the BNT-A, the treatment remains a risk for poorer oral condition and decreased self-cleaning ability. Caregivers and patients should cooperate with dentists, to improve oral hygiene and maintain good oral health [15, 34].
References
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601
Adler CH, Beach TG (2016) Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 31:1114–1119
Nutt JG (2016) Motor subtype in Parkinson’s disease: different disorders or different stages of disease? Mov Disord 31:957–961
Kadastik-Eerme L, Muldmaa M, Lilles S, Rosenthal M, Taba N, Taba P (2016) Nonmotor features in Parkinson’s disease: what are the most important associated factors? Park Dis 9:1–8
Kadastik-Eerme L, Rosenthal M, Paju T, Muldmaa M, Taba P (2015) Health-related quality of life in Parkinson’s disease: a cross-sectional study focusing on non-motor symptoms. Health Qual Life Outcomes 13:83
Marras C, Chaudhuri KR (2016) Nonmotor features of Parkinson’s disease subtypes. Mov Disord 31:1095–1102
Nicaretta DH, de Rosso ALZ, Maliska C, Costa MMB (2008) Scintigraphic analysis of the parotid glands in patients with sialorrhea and Parkinson’s disease. Parkinsonism Relat Disord 14:338–341
Kalf JG, Bloem BR, Munneke M (2012) Diurnal and nocturnal drooling in Parkinson’s disease. J Neurol 259:119–123
Karakoc M, Yon MI, Cakmakli GY, Ulusoy EK, Gulunay A, Oztekin N, Ak F (2016) Pathophysiology underlying drooling in Parkinson’s disease: oropharyngeal bradykinesia. Neurol Sci 37:1987–1991
Srivanitchapoom P, Pandley S, Hallett M (2014) Drooling in Parkinson’s disease: a review. Parkinsonism Relat Disord 20:1109–1118
Volonté MA, Porta M, Comi G (2002) Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurol Sci 23:121–122
Tumilasci OR, Cersósimo MG, Belforte JE, Micheli FE, Benarroch EE, Pazo JH (2006) Quantitative study of salivary secretion in Parkinson’s disease. Mov Disord 21:660–667
Fedorova T, Knudsen CS, Mouridsen K, Nexo E, Borghammer P (2015) Salivary acetylcholinesterase activity is increased in parkinson’s disease: a potential marker of parasympathetic dysfunction. Park Dis 2015:1–8
Hand AR, Frank ME (2014) Fundamentals of oral histology and physiology. Wiley-Blackwell, Hoboken, pp 224–285
Müller T, Palluch R, Jackowski J (2011) Caries and periodontal disease in patients with Parkinson’s disease. Spec Care Dentist 31(5):178–181
Bruno VA, Fox SH, Mancini D, Miyasaki JM (2016) Botulinum toxin use in refractory pain and other symptoms in parkinsonism. Can J Neurol Sci 43:697–702
Mostile G, Jankovic J (2009) Treatment of dysautonomia associated with Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S224–S232
Petracca M, Guidubaldi A, Ricciardi L, Ialongo T, Del Grande A, Mulas D, Di Stasio E, Bentivoglio AR (2015) Botulinum toxin A and B in sialorrhea: long-term data and literature overview. Toxicon 107:129–140
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, for the Movement Disorder Society UPDRS Revision Task Force (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Han DH, Kim MJ, Jun EJ, Kim JB (2013) The role of glutathione metabolism in cariogenic bacterial growth and caries in Korean children. Arch Oral Biol 58:493–499
Chifora I, Badeaa I, Chifora R, Tarmureb D, Popac D, Badeaa ME, Avrama R (2013) Healthier choices after saliva ph and buffering tests. Annals of West University of Timisoara. Series of Chemistry 22:19–30
DeBowes SL, Tolle SL, Bruhn AM (2013) Parkinson’s disease: considerations for dental hygienists. Int J Dent Hyg 11:15–21
Zlotnik Y, Balash Y, Korczyn AD, Giladi N, Gurevich T (2015) Disorders of the oral cavity in Parkinson’s disease and parkinsonian syndromes. Park Dis 2015:379482
Pfeiffer RF (2011) Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord 17:10–15
Bakke M, Larsen SL, Lautrup C, Karlsborg M (2011) Orofacial function and oral health in patients with Parkinson’s disease. Eur J Oral Sci 119:27–32
Sanchez P, Everett B, Salamonson Y, Ajwani S, George A (2017) Oral healthcare and cardiovascular disease: a scoping review of current strategies and implications for nurses. J Cardiovasc Nurs 32:E10–E20. https://doi.org/10.1097/JCN.0000000000000388
Äyräväinen L, Leirisalo-Repo M, Kuuliala A, Ahola K, Koivuniemi R, Meurman JH, Heikkinen AM (2017) Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in Finnish population. BMJ Open 7(1):e011916
Jost WH (2016) The option of sonographic guidance in Botulinum toxin injection for drooling in Parkinson’s disease. J Neural Transm 123:51–55
Dawes C (2007) Why does supragingival calculus form preferentially on the lingual surface of the 6 lower anterior teeth? Clin Pract 72:923–926
Ou R, Guo X, Wei Q, Cao B, Yang J, Song W, Chen K, Zhao B, Chen X, Shang H (2015) Diurnal drooling in Chinese patients with Parkinson’s disease. J Neurol Sci 353:74–78
Tiigimäe-Saar J, Taba P, Tamme T (2017) Does Botulinum neurotoxin type A treatment for sialorrhea change oral health? Clin Oral Investig 21:795–800
Tiigimäe-Saar J, Leibur E, Kolk A, Talvik I, Tamme T (2012) Use of botulinum neurotoxin A in uncontrolled salivation in children with cerebral palsy: a pilot study. Int J Oral Maxillofac Surg 41:1540–1545
Gómez-Caravaca MT, Cáceres-Redondo MT, Huertas-Fernández I, Vargas-González L, Carrillo F, Carballo M, Mir P (2015) The use of botulinum toxin in the treatment of sialorrhea in parkinsonian disorders. Neurol Sci 36:275–279
Pradeep AR, Singh SP, Martande SS, Raju AP, Rustagi T, Suke DK, Naik SB (2015) Clinical evaluation of the periodontal health condition and oral health awareness in Parkinson’s disease patients. Gerodontology 32:100–106
Funding
This study was supported by the Grants PUT1239 and IUT2-4 of the Estonian Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted in accordance with the national and international ethics standards, and was approved by the Research Ethics Committee of the University of Tartu (Protocol No. 221/T-15). Written informed consent was obtained from all participants. As the patients of group 1 received BNT-A injections, the study was approved by the Estonian State Agency of Medicines (Protocol No. 01-09.02.15; EudraCT number: 2015-000682-30).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tiigimäe-Saar, J., Tamme, T., Rosenthal, M. et al. Saliva changes in Parkinson’s disease patients after injection of Botulinum neurotoxin type A. Neurol Sci 39, 871–877 (2018). https://doi.org/10.1007/s10072-018-3279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3279-4