Abstract
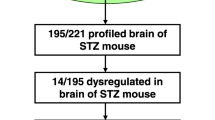
Diabetes mellitus is associated with dementia, but whether diabetes is associated with Alzheimer’s disease remains controversial. Alzheimer’s disease is characterized by amyloid beta aggregation. We hypothesized that genes, involved in amyloid beta degradation, may be altered due to diabetes and thus participate in progression of Alzheimer’s disease. Expression profiling of amyloid beta-degrading enzymes in streptozotocin-induced diabetic mice and their correlation with expression of amyloid precursor protein in hippocampus of Alzheimer’s disease patients were accessed. We found that matrix metalloproteinase 14 decreased in brain but not in other tissues of streptozotocin-induced diabetic mice, and was negatively correlated with expression of amyloid precursor protein in hippocampus of Alzheimer’s disease patients. These findings suggested matrix metalloproteinase 14 may link insulin-deficient diabetes to Alzheimer’s disease.



Similar content being viewed by others
References
Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Starr JM (2009) Age-associated cognitive decline. Br Med Bull 92(1):135–152
Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, Hofman A (2000) Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology 54(11 Suppl 5):S10–S15
Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283(12):1571–1577
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Ames D (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12(4):357–367
Coco M, Caggia S, Musumeci G, Perciavalle V, Graziano AC, Pannuzzo G, Cardile V (2013) Sodium L-lactate differently affects brain-derived neurothrophic factor, inducible nitric oxide synthase, and heat shock protein 70 kDa production in human astrocytes and SH-SY5Y cultures. J Neurosci Res 91(2):313–320
Puzzo D, Loreto C, Giunta S, Musumeci G, Frasca G, Podda MV, Palmeri A (2014) Effect of phosphodiesterase-5 inhibition on apoptosis and beta amyloid load in aged mice. Neurobiol Aging 35(3):520–531
Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Kivipelto M (2014) Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med 275(3):251–283
Chen SY, Hsu YM, Lin YJ, Huang YC, Chen CJ, Lin WD, Yang JS (2016) Current concepts regarding developmental mechanisms in diabetic retinopathy in Taiwan. Biomedicine 6(2):7
Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, Masaki K (2016) Diabetes is associated with cerebrovascular but not Alzheimer neuropathology. Alzheimer’s Dement
Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Kivipelto M (2010) Diabetes, Alzheimer disease, and vascular dementia a population-based neuropathologic study. Neurology 75(13):1195–1202
Arvanitakis Z, Schneider JA, Wilson RS, Li Y, Arnold SE, Wang Z, Bennett DA (2006) Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology 67(11):1960–1965
Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Haroutunian V (2005) Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol Ser A Biol Med Sci 60(4):471–475
Heitner J, Dickson D (1997) Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects: a retrospective postmortem immunocytochemical and histofluorescent study. Neurology 49(5):1306–1311
Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Iwaki T (2010) Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology 75(9):764–770
Vagelatos NT, Eslick GD (2013) Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev, mxs012
Ke YD, Delerue F, Gladbach A, Götz J, Ittner LM (2009) Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PLoS One 4(11):e7917
Devi L, Alldred MJ, Ginsberg SD, Ohno M (2012) Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer’s disease. PLoS One 7(3):e32792
Morales-Corraliza J, Wong H, Mazzella MJ, Che S, Lee SH, Petkova E, Mathews PM (2016) Brain-wide insulin resistance, tau phosphorylation changes, and hippocampal neprilysin and amyloid-β alterations in a monkey model of type 1 diabetes. J Neurosci 36(15):4248–4258
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Frangione B (2015) Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol 11(8):457–470
Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S (2008) SYMPOSIUM: clearance of Aβ from the brain in Alzheimer’s disease: Aβ-degrading enzymes in Alzheimer’s disease. Brain Pathol 18(2):240–252
Ito S, Ohtsuki S, Murata S, Katsukura Y, Suzuki H, Funaki M, Terasaki T (2014) Involvement of insulin-degrading enzyme in insulin-and atrial natriuretic peptide-sensitive internalization of amyloid-β peptide in mouse brain capillary endothelial cells. J Alzheimers Dis 38(1):185–200
Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Selkoe DJ (1998) Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem 273(49):32730–32738
Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H (2005) The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide. Eur J Neurosci 21(3):733–740
Zou K, Yamaguchi H, Akatsu H, Sakamoto T, Ko M, Mizoguchi K, Yanagisawa K (2007) Angiotensin-converting enzyme converts amyloid β-protein 1–42 (Aβ1–42) to Aβ1–40, and its inhibition enhances brain Aβ deposition. J Neurosci 27(32):8628–8635
Exley C, Korchazhkina OV (2001) Plasmin cleaves Aβ42 in vitro and prevents its aggregation into β-pleated sheet structures. Neuroreport 12(13):2967–2970
Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S (2000) Tissue plasminogen activator requires plasminogen to modulate amyloid-β neurotoxicity and deposition. J Neurochem 75(5):2172–2177
Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG (2000) Brain plasmin enhances APP α-cleavage and Aβ degradation and is reduced in Alzheimer’s disease brains. EMBO Rep 1(6):530–535
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8(2):205–216
Khokha R, Denhardt DT (1988) Matrix metalloproteinases and tissue inhibitor of metalloproteinases: a review of their role in tumorigenesis and tissue invasion. Invasion Metastasis 9(6):391–405
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Hanahan D (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737
Musumeci G, Loreto C, Clementi G, Fiore CE, Martinez G (2011) An in vivo experimental study on osteopenia in diabetic rats. Acta Histochem 113(6):619–625
Werb Z, Chin JR (1998) Extracellular matrix remodeling during morphogenesis. Ann N Y Acad Sci 857(1):110–118
Leonardi R, Loreto C, Barbato E, Caltabiano R, Lombardo C, Musumeci G, Muzio LL (2008) MMP-13 (collagenase 3) localization in human temporomandibular joint discs with internal derangement. Acta Histochem 110(4):314–318
Loreto C, Leonardi R, Musumeci G, Pannone G, Castorina S (2013) An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur J Histochem: EJH 57(2):e12
Leake A, Morris CM, Whateley J (2000) Brain matrix metalloproteinase 1 levels are elevated in Alzheimer’s disease. Neurosci Lett 291(3):201–203
Deb S, Gottschall PE (1996) Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with β-amyloid peptides. J Neurochem 66(4):1641–1647
Du H, Li P, Wang J, Qing X, Li W (2012) The interaction of amyloid β and the receptor for advanced glycation endproducts induces matrix metalloproteinase-2 expression in brain endothelial cells. Cell Mol Neurobiol 32(1):141–147
Liao MC, Van Nostrand WE (2010) Degradation of soluble and fibrillar amyloid β-protein by matrix metalloproteinase (MT1-MMP) in vitro. Biochemistry 49(6):1127–1136
Brkic M, Balusu S, Van Wonterghem E, Gorlé N, Benilova I, Kremer A, Libert C (2015) Amyloid β oligomers disrupt blood–CSF barrier integrity by activating matrix metalloproteinases. J Neurosci 35(37):12766–12778
Backstrom JR, Lim GP, Cullen MJ, Tökés ZA (1996) Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-β peptide (1–40). J Neurosci 16(24):7910–7919
Asahina M, Yoshiyama Y, Hattori T (2000) Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin Neuropathol 20(2):60–63
Castrogiovanni P, Trovato FM, Szychlinska MA, Nsir H, Imbesi R, Musumeci G (2016) The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol Histopathol 31(11):1183–1194
Concetta Aiello F, Maria Trovato F, Anna Szychlinska M, Imbesi R, Castrogiovanni P, Loreto C, Musumeci G (2017) Molecular links between diabetes and osteoarthritis: the role of physical activity. Curr Diabetes Rev 13(1):50–58
Hüttenrauch M, Brauss A, Kurdakova A, Borgers H, Klinker F, Liebetanz D, Wirths O (2016) Physical activity delays hippocampal neurodegeneration and rescues memory deficits in an Alzheimer disease mouse model. Transl Psychiatry 6(5):e800
Kjaer M, Langberg H, Miller BF, Boushel R, Crameri R, Koskinen S, Pedersen SG (2005) Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskelet Neuronal Interact 5(1):41–52
Nagase H (1997) Activation mechanisms of matrix metalloproteinases. Biol Chem 378(3–4):151–160
Mansford KRL, Opie L (1968) Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet 291(7544):670–671
D’Amico AG, Maugeri G, Reitano R, Bucolo C, Saccone S, Drago F, D’Agata V (2015) PACAP modulates expression of hypoxia-inducible factors in streptozotocin-induced diabetic rat retina. J Mol Neurosci 57(4):501–509
Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S (2000) Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 43(12):1528–1533
Schnedl WJ, Ferber S, Johnson JH, Newgard CB (1994) STZ transport and cytotoxicity: specific enhancement in GLUT2-expressing cells. Diabetes 43(11):1326–1333
Petryszak R, Keays M, Tang YA, Fonseca NA, Barrera E, Burdett T, Mannion O (2015) Expression atlas update—an integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Res 44(D1):D746–D752
Suzuki R, Lee K, Jing E, Biddinger SB, McDonald JG, Montine TJ, Kahn CR (2010) Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab 12(6):567–579
Van Lunteren E, Moyer M (2007) Oxidoreductase, morphogenesis, extracellular matrix, and calcium ion-binding gene expression in streptozotocin-induced diabetic rat heart. Am J Physiol Endocrinol Metab 293(3):E759–E768
Willsky GR, Chi LH, Liang Y, Gaile DP, Hu Z, Crans DC (2006) Diabetes-altered gene expression in rat skeletal muscle corrected by oral administration of vanadyl sulfate. Physiol Genomics 26(3):192–201
Altirriba J, Barbera A, Del Zotto H, Nadal B, Piquer S, Sánchez-Pla A, Gomis R (2009) Molecular mechanisms of tungstate-induced pancreatic plasticity: a transcriptomics approach. BMC Genomics 10(1):406
Salbaum JM, Kruger C, Zhang X, Delahaye NA, Pavlinkova G, Burk DH, Kappen C (2011) Altered gene expression and spongiotrophoblast differentiation in placenta from a mouse model of diabetes in pregnancy. Diabetologia 54(7):1909–1920
Franko A, von Kleist-Retzow JC, Neschen S, Wu M, Schommers P, Böse M, Huntgeburth M (2014) Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J Hepatol 60(4):816–823
Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL (2008) Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology 149(10):4928–4937
Baelde HJ, Eikmans M, Doran PP, Lappin DW, de Heer E, Bruijn JA (2004) Gene expression profiling in glomeruli from human kidneys with diabetic nephropathy. Am J Kidney Dis 43(4):636–650
Frederiksen CM, Højlund K, Hansen L, Oakeley EJ, Hemmings B, Abdallah BM, Gaster M (2008) Transcriptional profiling of myotubes from patients with type 2 diabetes: no evidence for a primary defect in oxidative phosphorylation genes. Diabetologia 51(11):2068–2077
Cangemi C, Skov V, Poulsen MK, Funder J, Twal WO, Gall MA, Parving HH (2011) Fibulin-1 is a marker for arterial extracellular matrix alterations in type 2 diabetes. Clin Chem 57(11):1556–1565
Marselli L, Thorne J, Dahiya S, Sgroi DC, Sharma A, Bonner-Weir S, Weir GC (2010) Gene expression profiles of beta-cell enriched tissue obtained by laser capture microdissection from subjects with type 2 diabetes. PLoS One 5(7):e11499
Pihlajamaki J, Boes T, Kim EY, Dearie F, Kim BW, Schroeder J, Goldfine AB (2009) Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab 94(9):3521–3529
Van Tienen FHJ, Praet SFE, De Feyter HM, van den Broek NM, Lindsey PJ, Schoonderwoerd KGC, van Loon LJC (2012) Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J Clin Endocrinol Metab 97(9):3261–3269
Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, Kiyohara Y (2014) Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb Cortex 24(9):2476–2488
Silva JA, Ferrucci DL, Peroni LA, Abrahao PG, Salamene AF, Rossa-Junior C, Stach-Machado DR (2012) Sequential IL-23 and IL-17 and increased Mmp8 and Mmp14 expression characterize the progression of an experimental model of periodontal disease in type 1 diabetes. J Cell Physiol 227(6):2441–2450
Thrailkill KM, Bunn RC, Fowlkes JL (2009) Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine 35(1):1–10
Catania JM, Chen G, Parrish AR (2007) Role of matrix metalloproteinases in renal pathophysiologies. Am J Physiol Ren Physiol 292(3):F905–F911
Chun TH, Inoue M, Morisaki H, Yamanaka I, Miyamoto Y, Okamura T, Weiss SJ (2010) Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes 59(10):2484–2494
Death AK, Fisher EJ, McGrath KC, Yue DK (2003) High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis 168(2):263–269
Kowluru RA, Kanwar M (2009) Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radic Biol Med 46(12):1677–1685
Tarallo S, Beltramo E, Berrone E, Dentelli P, Porta M (2010) Effects of high glucose and thiamine on the balance between matrix metalloproteinases and their tissue inhibitors in vascular cells. Acta Diabetol 47(2):105–111
Papazafiropoulou A, Perrea D, Moyssakis I, Kokkinos A, Katsilambros N, Tentolouris N (2010) Plasma levels of MMP-2, MMP-9 and TIMP-1 are not associated with arterial stiffness in subjects with type 2 diabetes mellitus. J Diabetes Complicat 24(1):20–27
van der Zijl NJ, Hanemaaijer R, Tushuizen ME, Schindhelm RK, Boerop J, Rustemeijer C, Diamant M (2010) Urinary matrix metalloproteinase-8 and-9 activities in type 2 diabetic subjects: a marker of incipient diabetic nephropathy? Clin Biochem 43(7):635–639
Thrailkill KM, Bunn RC, Moreau CS, Cockrell GE, Simpson PM, Coleman HN, Fowlkes JL (2007) Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care 30(9):2321–2326
Derosa G, D'Angelo A, Scalise F, Avanzini MA, Tinelli C, Peros E, Cicero AF (2007) Comparison between metalloproteinases-2 and-9 in healthy subjects, diabetics, and subjects with acute coronary syndrome. Heart Vessel 22(6):361–370
Ishibashi T, Kawaguchi M, Sugimoto K, Uekita H, Sakamoto N, Yokoyama K, Takeishi Y (2010) Advanced glycation end product-mediated matrix metallo-proteinase-9 and apoptosis via renin-angiotensin system in type 2 diabetes. J Atheroscler Thromb 17(6):578–589
Tinahones FJ, Coín-Aragüez L, Mayas MD, Garcia-Fuentes E, Hurtado-del-Pozo C, Vendrell J, El Bekay R (2012) Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol 12(1):4
Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42(9):1681–1681
Mann DMA, Tucker CM, Yates PO (1987) The topographic distribution of senile plaques and neurofibrillary tangles in the brains of non-demented persons of different ages. Neuropathol Appl Neurobiol 13(2):123–139
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7(2):331–356
Fukumoto H, Asami-Odaka A, Suzuki N, Shimada H, Ihara Y, Iwatsubo T (1996) Amyloid beta protein deposition in normal aging has the same characteristics as that in Alzheimer’s disease. predominance of A beta 42 (43) and association of A beta 40 with cored plaques. Am J Pathol 148(1):259
Funding
This work was supported by grants from the Ministry of Science and Technology in Taiwan (MOST105-2632-B-039-002, MOST 104-2320-B-039-009) and grants from China Medical University & Hospital (CMU105-S-39, DMR-101-065, DMR-105-085, CMU98-CT-25). This study is also supported in part by the Brain Diseases Research Center at China Medical University (BDRC 2017), and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.C and H.-P.L.; Methodology, J.C and H.-P.L.; Investigation, J.C and C.-C. L.; Writing—Original Draft, J.C and C.-C. L.; Writing—Review and Editing, M.-Y.C., W.-Y.L. and H.-P.L.; Funding Acquisition, W.-Y.L. and F.-J.T.; Resources, W.-Y.L. and F.-J.T.; Supervision, F.-J.T.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jack Cheng and Hsin-Ping Liu are co-first Authors.
Rights and permissions
About this article
Cite this article
Cheng, J., Liu, HP., Lee, CC. et al. Matrix metalloproteinase 14 modulates diabetes and Alzheimer’s disease cross-talk: a meta-analysis. Neurol Sci 39, 267–274 (2018). https://doi.org/10.1007/s10072-017-3166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3166-4