Abstract
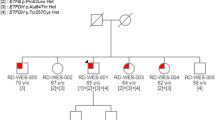
Late-onset multiple acyl-CoA dehydrogenase deficiency (MADD) with electron transfer flavoprotein dehydrogenase (ETFDH) gene mutations is the most common lipid storage myopathy (LSM) in China. Its clinical features vary widely and pose a challenge for diagnosis. We presented the significant clinical heterogeneity among three Chinese late-onset MADD patients with similar ETFDH genotype by collecting clinical information, muscle histology, and genetic analysis. Three novel compound heterozygous variants of ETFDH gene were identified: c.892C > T (p.Pro298Ser), c.453delA (p.Glu152ArgfsTer15), and c.449_453delTAACA (p.Leu150Ter). Moreover, all patients carried a hotspot mutation c.250G > A (p.Ala84Thr). Western blot analysis of the patients’ muscular tissue showed a significantly reduced ETFDH expression, and normal electron transfer flavoprotein A (ETFA) and electron transfer flavoprotein B (ETFB) expression. Two patients with similar genotypes (c.453delA and c.449_453delTAACA) presented a significant clinical heterogeneity. Among them, one exhibited muscle weakness and exercise intolerance as initial and major symptoms, and the other showed episodic recurrent gastrointestinal symptoms before a serious muscle weakness appeared in later life. The novel variants in ETFDH and the corresponding clinical features enrich the variant spectrum of late-onset MADD and provide a new insight into the genotype-phenotype relationship. Late-onset MADD should be included in differential diagnosis for adult myopathy along with chronic digestive disease.



Similar content being viewed by others
References
Burr ML, Roos JC, Ostör AJ (2008) Metabolic myopathies: a guide and update for clinicians. Curr Opin Rheumatol 20:639–647
Ohkuma A, Noguchi S, Sugie H, Malicdan MC, Fukuda T, Shimazu K, López LC, Hirano M, Hayashi YK, Nonaka I, Nishino I (2009) Clinical and genetic analysis of lipid storage myopathies. Muscle Nerve 39:333–342
Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, Frerman FE, Beresford MW, Dean JC, Cornelius N, Andersen O, Oldfors A, Holme E, Gregersen N, Turnbull DM, Morris AA (2007) ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain 130:2045–2054
Xi J, Wen B, Lin J, Zhu W, Luo S, Zhao C, Li D, Lin P, Lu J, Yan C (2014) Clinical features and ETFDH mutation spectrum in a cohort of 90 Chinese patients with late-onset multiple acyl-CoA dehydrogenase deficiency. J Inherit Metab Dis 37:399–404
Wen B, Dai T, Li W, Zhao Y, Liu S, Zhang C, Li H, Wu J, Li D, Yan C (2010) Riboflavin responsive lipid storage myopathy caused by ETFDH gene mutations. J Neurol Neurosurg Psychiatry 81:231–236
Wang ZQ, Chen XJ, Murong SX, Wang N, Wu ZY (2011) Molecular analysis of 51 unrelated pedigrees with late-onset multiple acyl-CoA dehydrogenation deficiency (MADD) in southern China confirmed the most common ETFDH mutation and high carrier frequency of c.250G > A. J Mol Med 89:569–576
Lan MY, Fu MH, Liu YF, Huang CC, Chang YY, Liu JS, Peng CH, Chen SS (2010) High frequency of ETFDH c.250G > A mutation in Taiwanese patients with late-onset lipid storage myopathy. Clin Genet 78:565–569
Wen B, Li D, Li W, Zhao Y, Yan C (2015) Multiple acyl-CoA dehydrogenation deficiency as decreased acyl-carnitine profile in serum. Neurol Sci 36:853–859
Zhang Jian, Frerman Frank E, Kim Jung-Ja P (2006) Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc Natl Acad Sci USA 103:16212–16217
Olsen RK, Pourfarzam M, Morris AA, Dias RC, Knudsen I, Andresen BS, Gregersen N, Olpin SE (2004) Lipid-storage myopathy and respiratory insufficiency due to ETFQO mutations in a patient with late-onset multiple acyl-CoA dehydrogenation deficiency. J Inherit Metab Dis 27:671–678
Angle B, Burton BK (2008) Risk of sudden death and acute life-threatening events in patients with glutaric acidemia type II. Mol Genet Metab 93:36–39
Yotsumoto Y, Hasegawa Y, Fukuda S, Kobayashi H, Endo M, Fukao T, Yamaguchi S (2008) Clinical and molecular investigations of Japanese cases of glutaric acidemia type 2. Mol Genet Metab 94:61–67
Beresford MW, Pourfarzam M, Turnbull DM, Davidson JE (2006) So doctor, what exactly is wrong with my muscles? Glutaric aciduria type II presenting in a teenager. Neuromuscul Disord 16:269–273
Olsen RK, Andresen BS, Christensen E, Bross P, Skovby F, Gregersen N (2003) Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat 22:12–23
Cornelius N, Frerman FE, Corydon TJ, Palmfeldt J, Bross P, Gregersen N, Olsen RK (2012) Molecular mechanisms of riboflavin responsiveness in patients with ETF-QO variations and multiple acyl-CoA dehydrogenation deficiency. Hum Mol Genet 21:3435–3448
Zhu M, Zhu X, Qi X, Weijiang D, Yu Y, Wan H, Hong D (2014) Riboflavin-responsive multiple Acyl-CoA dehydrogenation deficiency in 13 cases, and a literature review in mainland Chinese patients. J Hum Genet 59:256–261
Acknowledgments
We thank the patients and their family members for their cooperation. This work was supported by Grants from the National Natural Science Foundation of China (81271254, Beijing), National Key Clinical Specialty Discipline Construction Program, and Fujian Key Clinical Specialty Discipline Construction Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Grants from the National Natural Science Foundation of China (Grant Number: 81271254), National Key Clinical Specialty Discipline Construction Program, and Fujian Key Clinical Specialty Discipline Construction Program.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
H. Fu and X. Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fu, Hx., Liu, Xy., Wang, Zq. et al. Significant clinical heterogeneity with similar ETFDH genotype in three Chinese patients with late-onset multiple acyl-CoA dehydrogenase deficiency. Neurol Sci 37, 1099–1105 (2016). https://doi.org/10.1007/s10072-016-2549-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2549-2