Abstract
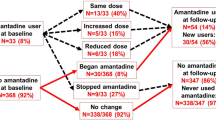
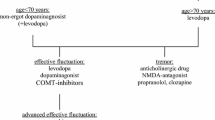
To assess over a period of 9 months in a sample of Italian Parkinson’s disease (PD) patients reasons leading the neurologist to modify dopaminergic treatment and patients’ causes of dissatisfaction with ongoing therapy. To evaluate the influence of disease severity on therapy persistence. A disease severity balanced sample of PD patients with stable anti-parkinsonian drugs (APD) treatment was enrolled and evaluated every 3 months. Patients requiring APD treatment modifications were discontinued from the study. The probability to modify APD treatment is greater for higher motor (UPDRS scores) and non-motor symptoms (NMSS score) severity. Both from neurologist’s and patient’s perspective, motor symptoms were the main determinants underlying APD treatment modifications. Non-motor symptoms were cause of dissatisfaction with ongoing APD treatment for 52 % of the patients, while only 36 % of the neurologists considered these as valid reasons for therapy change. REASON is the first study in PD patients that prospectively examined reasons driving APD treatment changes. Results show that the disease severity significantly increases the probability of APD treatment change. Patients attribute greater relevance than neurologists to non-motor symptoms as reason requiring treatment changes. This confirms that patient and neurologist perceptions only partially overlap.


Similar content being viewed by others
References
Poewe W (2009) Treatments for Parkinson disease—past achievements and current clinical needs. Neurology 72:S65–S73
Grosset D, European PD Therapy Compliance Study Group (2010) Therapy adherence issues in Parkinson’s disease. J Neurol Sci 289(1–2):115–118
Tinazzi M, Abbruzzese G, Antonini A, Ceravolo R, Fabbrini G, Lessi P, Barone P, REASON Study Group (2013) Reasons driving treatment modification in Parkinson’s disease: results from the cross-sectional phase of the REASON study. Parkinsonism Relat Disord 19:1130–1135
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observation. J Am Stat Assoc 53:457–481
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. MovDisord 25:2649–2653
Fargel M, Grobe B, Oesterle E, Hastedt C, Rupp M (2007) Treatment of Parkinson’s disease: a survey of patients and neurologists. Clin Drug Investig 27:207–218
Tarrants ML, Denarié MF, Castelli-Haley J, Millard J, Zhang D (2010) Drug therapies for Parkinson’s disease: a database analysis of patient compliance and persistence. Am J GeriatrPharmacother 8:374–383
Acknowledgments
This research was sponsored by a grant from Boehringer-Ingelheim. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all aspects of manuscript development. We are grateful to MediData (Modena, Italy) for data collection and statistical analysis and to Sara Rizzoli and Shalom Haggiag for help in writing the manuscript.
Ethical standards
Each patient signed written informed consent and each local institutional ethics committee approved the study.
Conflict of interest
Patrizia Lessi is an employee of Boehringer-Ingelheim. Alessandra Ori is an employee of Medidata srl. Lucia Simoni is an employee of Medidata srl. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the REASON study group. The members of REASON study group are listed in “Appendix”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: The REASON study group
Appendix: The REASON study group
Steering Committee:
-
Giovanni Abruzzese, Università degli Studi di Genova, Genova
-
Angelo Antonini, Ospedale IRCCS San Camillo, Venezia Lido e Università di Padova
-
Paolo Barone, Università degli Studi di Salerno Schola Medica Salernitana, Salerno
-
Roberto Ceravolo, Ospedale Santa Chiara, Pisa
-
Giovanni Fabbrini, Sapienza Università di Roma, and Neuromed Institute, Pozzilli (IS)
-
Michele Tinazzi,Unità Operativa di Neurologia/Dipartimento di Scienze Neurologiche, Neuropsicologiche, Morfologiche e Motorie, Azienda Ospedaliera-Universitaria di Verona
Participating centres:
-
M. A. B. Melone, C. Schettino, G. Capaldo, Az. Osp.Univ. Seconda Universtà di Napoli (Napoli)
-
F. Iemolo, E. Sanzaro, Ospedale Civile R. Guzzardi (Vittoria);
-
M. G. Ceravolo, M. Capecci, E. Andrenelli, Az. Ospedali Riuniti (Ancona);
-
F. E. Pontieri, C. Pellicano, D. Benincasa, Az. Osp. Sant’Andrea (Roma);
-
G. Fabbrini, S. Pietracupa, A. Latorre, Sapienza Università di Roma;
-
G. Tedeschi, A. Tessitore, A. Giordano, Seconda Università Federico II (Napoli);
-
U. Bonuccelli, D. Frosini, F. Vanelli, Ospedale Santa Chiara (Pisa);
-
G. Comi, M. A. Volonté, F. Spagnolo, Fondazione Centro San Raffaele (Milano);
-
A. Scaglioni, G. Abrignani, Ospedale di Vaio (Fidenza);
-
G. Abbruzzese, L. Avanzino, T. Tamburini, Università degli Studi di Genova (Genova);
-
A. Antonini, S. Facchini, R. Biundo, Ospedale San Camillo (Venezia Lido);
-
M. C. Altavista, C. Roberti, Ospedale San Filippo Neri (Roma);
-
T. Avarello, Az. Osp. Riuniti Villa Sofia-Cervello (Palermo);
-
G. Bono, G. Riboldazzi, S. leva, Ospedale di Circolo e Fondazione Macchi (Varese);
-
M. Del Sette, E.Carabelli, E. Traverso, Ospedale Sant’ Andrea (La Spezia);
-
R. Michelucci, S. Nassetti, E.Pasini, Ospedale Bellaria (Bologna);
-
A. Padovani, E.Cottini, B. Bigni, Az. Osp. Spedali Civili (Brescia);
-
S. Ruggieri, N. Modugno, M. Fischetti, Istituto Mediterraneo Neuromed (Pozzilli);
-
A. Stefani, M. Pierantozzi, M. Stampanoni Bassi, Policlinico Tor Vergata (Roma);
-
M. Tinazzi, S. Ottaviani, D. Ajena, Dipartimento di Scienze Neurologiche e del movimento, Università di Verona, Italy;
-
G. Trianni, F. My, M. Caggiula, Ospedale Vito Fazzi (Lecce);
-
G. Valenti, S. Grioli, I. La Farina, Ospedale Garibaldi (Catania);
-
S. Zambito Marsala, C. Marchini, M. Gioulis, Ospedale San Martino (Belluno);
-
G. Asteggiano, M. R. L’Episcopo, E. Saracco, Ospedale San Lazzaro (Alba);
-
P. Barone, M. Picillo, M. Moccia, Università Federico II Napoli (Napoli);
-
M. Onofrj, A. Thomas, Fondazione Università Gabriele D’Annunzio (Chieti);
-
A. Denaro, Istituto Chirurgico Ortopedico Traumatologico (Latina);
-
C. Marini, F. De Santis, V. Spagnoli, Ospedale di Avezzano (Avezzano);
-
R. L’Erario, P. Passadore, Ospedale S. Maria della Misericordia (Rovigo);
-
E. Belgrado, M. Mucchiut, Az. Osp. Univ. S. Maria della Misericordia (Udine);
-
A. Priori, F. Cogiamanian, Fondazione Ospedale Maggiore Policlinico (Milano);
-
A. Marchet, Ospedale Martini (Torino)
Sponsorship:
-
BoehringerIngelheim, Milano, Italy
Project Management, Statistical Analyses and data management:
-
MediData Studi e Ricerche, Modena: G. Castegnaro, A. Ori, S. Pirondi, S. Rizzoli, B. Roncari, S. Sala, S. Sgarbi, L. Simoni, F. Trevisan, L. Zanoli.
Rights and permissions
About this article
Cite this article
Abbruzzese, G., Barone, P., Ceravolo, R. et al. Clinical variables associated with treatment changes in Parkinson’s disease: results from the longitudinal phase of the REASON study. Neurol Sci 36, 935–943 (2015). https://doi.org/10.1007/s10072-014-2060-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-2060-6