Abstract
The efficiency of aposematic colouration of prey is based on the innate bias or facilitation of avoidance learning of predators. In many toxic bufonids, larvae are uniformly black, which is considered a warning signal. We compared fish predation on normal (black) and ‘transient albino’ (greyish) common toad Bufo bufo tadpoles that did not differ in toxicity or activity. In a two-stage experiment, each fish was presented with tadpoles of one colour in the first trial and the other colour in a subsequent trial. While tadpoles sampled by fish were typically not ingested, some died from injuries. The attack rate did not differ between tadpole phenotypes nor trials, irrespective of which phenotype was the first exposed to the fish. However, during the second trial, the sampled tadpoles, independent of colouration, were mouthed by fish for shorter periods and tadpole mortality decreased. The duration of mouthing also declined with an increasing number of attacks during subsequent trials. We conclude that in single-species prey populations, black tadpole colouration is not a warning signal as it does not accelerate predator learning about prey unprofitability. Our results indicate that with growing experience, predators sample potentially toxic prey more cautiously. This may explain why natural selection does not eliminate aposematic morphs even if predators continuously sample conspicuous prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aposematic traits, like distinctive colouration, pattern, odour or sound, are warning signals to reduce predation risk. Such signals must be conspicuous before an attack begins and are considered a primary defence mechanism of the prey, advertising its unprofitability to predators. The features evoking aversion in the predator are considered secondary defences (Ruxton et al. 2004). A predator may either show innate bias against warning traits (Sillén-Tullberg 1985; Marples and Roper 1996; Rowe and Guilford 1996; Lindström et al. 1999) or learns to avoid a given type of prey due to the combination of both sets of traits (Sillén-Tullberg 1985; Speed 2000; Gamberale-Stille and Guilford 2004). In a learning process, the chances of the unpalatable prey being injured or killed should decrease (Ruxton et al. 2004; Rojas et al. 2017). However, predator naivety and/or deceptive (auto)mimicry result in predator uncertainty towards prey, driving the need to verify palatability. Predator learning via prey sampling (Skelhorn and Rowe 2006) poses an evolutionary problem of the aposematic prey's costs of educating the predators, especially as conspicuous traits increase prey detection rate (Wiklund and Järvi 1982; Ruxton et al. 2004; Puurtinen and Kaitala 2006; Skelhorn and Rowe 2006).
Conspicuous colours, such as black, red or yellow and their combinations, may have a warning function (Schuler and Roper 1992; D’Heursel and Haddad 1999) because they provide a high contrast against natural backgrounds (Gamberale-Stille 2001; Stevens and Ruxton 2012). In many toad species (Bufonidae) that possess chemical defences and are avoided by vertebrate predators, especially fish (Daly et al. 1987; Letnic et al. 2008; Kowalski et al. 2018), tadpoles are uniformly black. This black colouration is considered to be aposematic (Peterson and Blaustein 1991; Wells 2013), although alternative explanations have also been discussed (Guilford 1988). The effectiveness of black as an aposematic colouration of tadpoles may vary depending on the predator identity (D’Heursel and Haddad 1999; Gontijo et al. 2018). The potential aposematic function of contrasting colours is poorly understood in aquatic organisms as the refractive index of water is significantly different to air. Vision below the water surface depends on its properties, including transparency and light intensity (Vogel and Beauchamp 1999; Mazur and Beauchamp 2003). Black is assumed to be conspicuous under wide light conditions underwater (Levine et al. 1980; but see: Kinney et al. 1967), so it has the potential to act as an aposematic signal in aquatic animals. Fishes, the dominant aquatic predators to whom bufonid tadpoles appear invulnerable, have a well-developed sense of sight and see more colours than humans (Neumeyer 1992; Neumeyer and Mora-Ferrer 2001).
Our aim was to understand how predatory fish avoid unpalatable prey, in particular, whether black colouration of bufonid tadpoles is an aposematic signal. Experimental testing of the role of potential warning colouration in living aquatic animals is challenging because of difficulties with non-invasively manipulating animal colouration in aquatic environments. We circumvented this issue in predation trials using ‘transient albino’ (non-uniformly greyish coloured) and normal black tadpoles of the common toad Bufo bufo Linnaeus, 1758 that did not differ in their level of chemical defences. We predicted that if black colouration was a genuine warning signal, the fish would more rapidly learn to avoid black-coloured prey than the greyish, but similarly unpalatable, conspecifics. We compared the frequency of fish attacks on the two phenotypes in two consecutive trials (tadpoles of each phenotype were separately presented to fish in one of the trials), prey handling time and tadpole survival during each trial.
Materials and methods
The experiment
Large portions of two freshly laid egg strings of the common toad, one normally (black) coloured and one albino (white), were collected from a suburban pond near Poznań (52° 20′13.8″ N 16° 58′47.2″ E) and separately stored in aged tap water. After hatching, 100 tadpoles were randomly selected for each phenotype. The two groups were reared independently under uniform conditions in 100-L containers up to Gosner stage 25 (Gosner 1960); the same group sizes were used to standardise toxin production by tadpoles in response to conspecific density (Bókony et al. 2018).
We used the wild phenotype of goldfish, Carassius auratus Linnaeus, 1758, as a predator. Carassius auratus is a cyprinid native to Eastern Asia but widely introduced elsewhere (Savini et al. 2010). The goldfish is a generalist forager with a varied diet, including plankton, bottom-dwelling invertebrates and amphibian larvae (Monello and Wright 2001). Goldfish presence can affect amphibians in complex ways, invoking strong non-consumptive effects (Winandy and Denoël 2013, 2015). Owing to its omnivorous diet and ecosystem engineering abilities, C. auratus is an ecologically relevant model species representative of a large group of widely spread carp fishes, such as Carassius spp. and Cyprinus spp. (Richardson et al. 1995; Kloskowski 2009; Huang et al. 2020). Colour vision in this species is tetrachromatic (Neumeyer 1992). The goldfish (age 1 + fish) were obtained from a fish retailer. The fish were reared in semi-natural ponds without experience with common toad tadpoles (the only amphibian with black and toxic larvae in the region) until their first winter, after which they were kept in artificial conditions. The mean total length of the fish was 89.3 ± 1.5 mm (mean ± SE). At this size cyprinid fishes attain the ability to prey on freely-moving tadpoles in mid and late developmental stages (cf. Kloskowski 2009); hence 1 + spring is the period goldfish learn about the palatability of tadpoles in natural conditions.
For one week before the experiment, the fish were stored outdoors in a 120 × 100 cm2 tank and fed granulated feed and Chironomidae bloodworm larvae ad libitum. Twenty-four hours before the experiment, the goldfish were placed individually in plastic 39 × 28 × 14 cm plastic containers filled with approximately 10 L of aged tap water (temperature 18 °C), the bottom covered with commercial aquarium sand. To standardize hunger levels, fish were not fed during this period, except for receiving three bloodworm larvae of a similar size one hour before the trials. Five tadpoles of the same phenotype (either black or albino) were introduced into each container. As the transient albino tadpoles darken progressively over time (Henle et al. 2017), they were greyish at Gosner stage 25, thus, resembling non-aposematic tadpoles (Peterson and Blaustein 1991; Wells 2013). Throughout development, the differences in body colouration between the phenotypes were visible to a human observer (see Suppl. I). Using earlier stage tadpoles would provide larger differences in body colouration between groups; however, white colouration could also potentially function as an aposematic signal. Experimental treatments consisted of two subsequent 3-h trials, 10 min apart. Each tested fish was presented with both prey phenotypes (either first with albino and then black tadpoles or the reverse sequence, 9 fish individuals per each sequence of tadpole phenotype presentation, double trials, altogether 36 trials). Fish and tadpole behaviour was recorded using a Sony HDR-AS50 camera. The number of fish attacks (tadpole captures) during each trial was counted. The duration of prey handling (“mouthing”) was assessed using a stopwatch.
Since prey activity can alter predation rates (Gunzburger and Travis 2005), tadpole activity was assessed by recording the number of tadpoles swimming at the moment of observation. Activity counts were done near the beginning and in the middle of trials, each consisting of five repeated counts every minute from 15 to 19 min after trial onset, and 91 to 95 min, respectively. The mean proportion of active to non-active tadpoles at the beginning and in the middle of the trial was used in the analyses.
Analysis of toxin content
In toad tadpoles, the antipredator defences are based on bufadienolides and proteins present in the skin (Lawler and Hero 1997; Crossland and Alford 1998; Crossland 2001; Üveges et al. 2017; Bókony et al. 2018; Kowalski et al. 2018). Liquid chromatography-electrospray ionisation tandem mass spectrometry was applied to identify the five most common and abundant bufadienolides (bufalin, bufotalin, cinobufagin, cinobufotalin and resibufogenin; for details, see Suppl. I). Mass-corrected bufadienolide quantity was calculated by dividing the concentration of each bufadienolide by the dry mass of individual tadpoles. For analyses, the values of all compounds were summed to estimate the total amount of bufadienolides per individual (Bókony et al. 2018).
Statistical analysis
Linear mixed models were applied to assess the predatory behaviour of fish and tadpole survival. As the same fish individuals were used twice during the trials, fish identity was fitted as a random term. In all preliminary models, the colouration of tadpoles (black or albino), trial order (first or second trial) and sequence of tadpole phenotype presentation (which phenotype was presented first) were entered as fixed factors. Frequency of fish attacks on tadpoles (the total number of attacks during the trial) and duration of mouthing the prey were assessed using residual maximum likelihood models (REML). Residual plots were visually evaluated to ensure that each dataset met the assumption of normally distributed residual errors. The survival of tadpoles was analysed using generalised linear mixed models (GLMM) with a logit link and binomial distribution. The number of tadpole survivors was treated as a binomial response; the initial number of tadpoles constituted the binomial denominator. In the REML models, significance of the fixed terms was determined by the F statistics, and in the binomial models, by the Wald test. Model estimates were based on full models, except that the sequence of phenotype presentation and interaction terms (all P ≥ 0.275) were omitted due to non-significance. However, we also report minimal (backward simplified) models (cf. Forstmeier and Schielzeth 2011) when removing a highly insignificant predictor changed the significance value of another predictor to P < 0.05. All statistics were run in GenStat 15.1 (VSN International Ltd).
Results
No significant differences were found between black and albino tadpoles in the total amount of the five analysed bufadienolides per body mass (t test, t18 = 1.65, P = 0.115, mean ± SE 654.0 ± 56.4 µg/g vs 507.6 ± 68.2 µg/g, respectively). Also, black and albino tadpoles did not differ in activity levels at the beginning (t34 = 1.35, P = 0.187) or the end of trials (t34 = 0.41, P = 0.683).
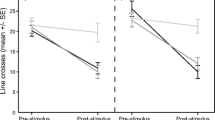
The fish attacked tadpoles in all of the trials (range 7–89 attacks per trial). The frequency of fish attacks did not differ between tadpole phenotypes nor for trial order (first or second) (Table 1, Fig. 1). The duration of prey handling (Fig. 2; post-hoc least-significant-difference (LSD) test showed that the effect was mainly explained by a strong decline in the duration of mouthing of the albino tadpoles) and the mortality of tadpoles, while not affected by tadpole phenotype, were lower in the second than the first trial (Table 1). As tadpole phenotype did not affect prey handling time by fish, an additional GLMM was run on the data from combined trials with the sequence of attacks (omitting a small number of immediately repeated attacks on the same prey) as a single fixed factor. The duration of prey mouthing declined with an increasing number of attacks during subsequent trials (F1, 871.5 = 7.45, P = 0.006). Most tadpole deaths were due to injuries from fish attacks (in total, ten during the first trial and three during the second), and only one tadpole was consumed.
Discussion
Avoiding toxic prey can significantly increase individual fitness; hence, quick yet efficient learning about aposematism should be adaptive in predators (Glendinning 2007; Rowland et al. 2017). Aposematic signals may accelerate this learning process to the advantage of both predator and prey (D’Heursel and Haddad 1999; Mappes et al. 2005; Ruxton et al. 2008). In our study, the black colouration of tadpoles did not significantly reduce the attack rate and the prey handling time by fish predators relative to the greyish (albino) tadpoles. We infer that when prey populations consist of a single species, the black body colour does not function as a warning signal in the aquatic environment. We did not test the alternative aposematic function of the black colouration, i.e., whether it would be effective in discriminating toxic tadpoles from undefended mimics for a predator; however, the effects of visual signals and possible chemical species-recognition cues would need to be separated (Holen 2013) to determine which of these factors is more important in model-mimic discrimination. Here, given their similar toxin levels, we assumed that any chemical warning signals did not differ between the two phenotypes of this species.
Fish predators are known to avoid unpalatable bufonid tadpoles (Voris and Bacon 1966; Kruse and Stone 1984; Lawler and Hero 1997), but the processes managing their recognition of prey defences remain poorly understood. The rate of predator learning and the survival of unpalatable tadpoles may depend on the relative abundance of otherwise similar but palatable prey, as well as the predator hunger levels (Nelson et al. 2011; Kaczmarek et al. 2018; Kaczmarek et al. 2020; cf. Lindström et al. 2004; Rowland et al. 2010). We found that learning in fish was based on tasting prey (see also: Nelson et al. 2011; Nomura et al. 2011), with shorter mouthing durations in the second compared to the first trial despite no change in the frequency of fish attacks. Additionally, the overall duration of prey mouthing declined with an increasing number of attacks during subsequent trials. The short experiment duration did not allow inference of long-term retention of the memory of toxic prey. The decrease in prey handling time was not necessarily a result of learning; when sampling the same group of toad tadpoles, the predators may have been exposed to increasing amounts of defensive toxins as tadpoles were repeatedly captured and possibly injured. However, we observed a decrease in mouthing time from the first to the second trial, despite a fresh group of tadpoles being used in the second trial. This indicates that the change in fish behaviour was based on learning and not a simple aversion to increasingly toxic prey. A continuous yet cautious sampling of prey and rejecting the unpalatable individuals has been documented as a way to discriminate between automimics and models in aposematic systems (Guilford 1994; Gamberale-Stille and Guilford 2004; Holen 2013). An obvious benefit for the predator is that this strategy reduces the exposure to prey toxins (Gamberale-Stille and Guilford 2004) and limits the opportunities for cheating by palatable mimics (Skelhorn and Rowe 2006). However, prey is more likely to escape if predators treat it with caution (Sherratt 2002; Yamazaki et al. 2020). The costs for unpalatable prey when being attacked and tasted are still unclear, as well as the fitness value of conspicuous traits if they do not deter predators from sampling the prey (Rowland et al. 2010). In laboratory experiments, vertebrate predators have been observed to taste and reject bufonid tadpoles, apparently unharmed (Peterson and Blaustein 1991; D’Heursel and Haddad 1999; Crossland 2001; Grasso et al. 2010). However, in contrast to animals morphologically adapted to being handled by predators (Sillén-Tullberg 1985; Skelhorn and Rowe 2006; Wang et al. 2018), anuran tadpoles are highly sensitive to handling due to their delicate skin, with even slight injuries possibly leading to mortality during repeated attacks (Duellman and Trueb 1986). In the present study, a few toad tadpoles died after being captured, although they were not consumed. However, the decrease in the duration of prey mouthing suggests that experienced fish treat tadpoles with increasing caution (Nelson et al. 2011; see also: Paradise and Stamp 1991; Hotová Svádová et al. 2013), which could mitigate the injury risk for unpalatable prey (Sillén-Tullberg et al. 1982; Paradise and Stamp 1991). Indeed, toad tadpoles had higher survival rates during the second than the first trial. Decreasing recognition time (Hughes 1979) and increasing caution may explain the persistence of the unpalatable prey when predators choose to sample them despite their conspicuous colouration, i.e., why natural (individual) selection does not act against aposematic prey (see also: Wiklund and Järvi 1982). Occurrence at high densities (Gazzola and Van Buskirk 2015) and gregariousness of unpalatable prey (Waldman and Adler 1979; Svádová et al. 2014) may provide fitness benefits additional to improved learning by predators (Skelhorn et al. 2016), in that costs of sampling by predators are spread over more conspecifics (density-dependent dilution; Speed 2000; Rowland et al. 2010).
Data availability
Raw data from the study can be accessed in the electronic supplementary material S2.
References
Bókony V, Üveges B, Móricz ÁM, Hettyey A (2018) Competition induces increased toxin production in toad larvae without allelopathic effects on heterospecific tadpoles. Funct Ecol 32:667–675. https://doi.org/10.1111/1365-2435.12994
Crossland MR (2001) Ability of predatory native Australian fishes to learn to avoid toxic larvae of the introduced toad Bufo marinus. J Fish Biol 59:319–329. https://doi.org/10.1006/jfbi.2001.1640
Crossland MR, Alford RA (1998) Evaluation of the toxicity of eggs, hatchlings and tadpoles of the introduced toad Bufo marinus (Anura: Bufonidae) to native Australian aquatic predators. Austral Ecol 23:129–137. https://doi.org/10.1111/j.1442-9993.1998.tb00711.x
D’Heursel A, Haddad CFB (1999) Unpalatability of Hyla semilineata tadpoles (Anura) to captive and free-ranging vertebrate predators. Ethol Ecol Evol 11:339–348. https://doi.org/10.1080/08927014.1999.9522818
Daly J, Myers CW, Whittaker N (1987) Further classification of skin alkaloids from neotropical poison frogs (Dendrobatidae), with a general survey of toxic/noxious substances in the amphibia. Toxicon 25:1023–1095
Duellman WE, Trueb L (1986) Chapter 7. Metamorphosis. Biology of amphibians. McGraw Hill Book Companz, New York, pp 173–194
Forstmeier W, Schielzeth H (2011) Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55. https://doi.org/10.1007/s00265-010-1038-5
Gamberale-Stille G (2001) Benefit by contrast: an experiment with live aposematic prey. Behav Ecol 12:768–772. https://doi.org/10.1093/beheco/12.6.768
Gamberale-Stille G, Guilford T (2004) Automimicry destabilizes aposematism: predator sample-and-reject behaviour may provide a solution. Proc R Soc B Biol Sci 271:2621–2625. https://doi.org/10.1098/rspb.2004.2893
Gazzola A, Van Buskirk J (2015) Isocline analysis of competition predicts stable coexistence of two amphibians. Oecologia 178:153–159. https://doi.org/10.1007/s00442-015-3273-y
Glendinning JI (2007) How do predators cope with chemically defended foods? Biol Bull 213:525–266. https://doi.org/10.2307/25066643
Gontijo ASB, Espanha J, Eterovick PC (2018) Is tadpole coloration adaptive against bird predation? Acta Ethol 21:69–79. https://doi.org/10.1007/s10211-018-0285-8
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grasso RL, Coleman RM, Davidson C (2010) Palatability and antipredator response of Yosemite Toads (Anaxyrus canorus) to nonnative Brook Trout (Salvelinus fontinalis) in the Sierra Nevada Mountains of California. Copeia 2010:457–462. https://doi.org/10.1643/CH-09-033
Guilford T (1988) The evolution of conspicuous coloration. Am Nat 131:7–21. https://doi.org/10.1086/284764
Guilford T (1994) “Go-slow” signalling and the problem of automimicry. J Theor Biol 170:311–316. https://doi.org/10.1006/jtbi.1994.1192
Gunzburger MS, Travis J (2005) Critical literature review of the evidence for unpalatability of amphibian eggs and larvae. J Herpetol 39:547–571. https://doi.org/10.1670/1-05A.1
Henle K, Dubois A, Vershinin V (2017) A review of anomalies in natural populations of amphibians and their potential causes. In: Henle K, Dubois A (eds) Studies on anomalies in natural populations of amphibians. Martensiella, Mannheim, pp 57–164
Holen ØH (2013) Disentangling taste and toxicity in aposematic prey. Proc R Soc B Biol Sci 280:20122588. https://doi.org/10.1098/rspb.2012.2588
Hotová Svádová K, Exnerová A, Kopečková M, Štys P (2013) How do predators learn to recognize a mimetic complex: experiments with naive great tits and aposematic heteroptera. Ethology 119:814–830. https://doi.org/10.1111/eth.12121
Huang Y, Mei X, Rudstam LG, Taylor WD, Urabe J, Jeppesen E, Liu Z, Zhang X (2020) Effects of crucian carp (Carassius auratus) on water quality in aquatic ecosystems: an experimental mesocosm study. Water 12:1444. https://doi.org/10.3390/w12051444
Hughes RN (1979) Optimal diets under the energy maximization premise: the effects of recognition time and learning. Am Nat 113:209–221. https://doi.org/10.1086/283380
Kaczmarek JM, Kaczmarski M, Mazurkiewicz J, Kloskowski J (2018) A matter of proportion? Associational effects in larval anuran communities under fish predation. Oecologia. https://doi.org/10.1007/s00442-018-4141-3
Kaczmarek JM, Kaczmarski M, Mazurkiewicz J, Kloskowski J (2020) Numbers, neighbors, and hungry predators: what makes chemically defended aposematic prey susceptible to predation? Ecol Evol 10:13705–13716. https://doi.org/10.1002/ece3.6956
Kinney JA, Luria SM, Weitzman DO (1967) Visibility of colors underwater. J Opt Soc Am 57:802–809. https://doi.org/10.1364/JOSA.57.000802
Kloskowski J (2009) Size-structured effects of common carp on reproduction of pond-breeding amphibians. Hydrobiologia 635:205–213. https://doi.org/10.1007/s10750-009-9912-8
Kowalski K, Marciniak P, Rosiński G, Rychlik L (2018) Toxic activity and protein identification from the parotoid gland secretion of the common toad Bufo bufo. Comp Biochem Physiol Part C Toxicol Pharmacol 405:43–52. https://doi.org/10.1016/j.cbpc.2018.01.004
Kruse KC, Stone BS (1984) Largemouth bass (Micropterus salmoides) learn to avoid feeding on toad (Bufo) tadpoles. Anim Behav 32:1035–1039
Lawler KL, Hero JM (1997) Palatability of Bufo marinus tadpoles to a predatory fish decreases with development. Wildl Res 24:327–334. https://doi.org/10.1071/WR96089
Letnic M, Webb JK, Shine R (2008) Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol Conserv 141:1773–1782. https://doi.org/10.1016/j.biocon.2008.04.031
Levine JS, Lobel PS, MacNichol EF (1980) Visual communication in fishes. Environmental physiology of fishes. Plenum, New York, pp 447–475
Lindström L, Alatalo RV, Mappes J (1999) Reactions of hand-reared and wild-caught predators toward warningly colored, gregarious, and conspicuous prey. Behav Ecol 10:317–322. https://doi.org/10.1093/beheco/10.3.317
Lindström L, Alatalo RV, Lyytinen A, Mappes J (2004) The effect of alternative prey on the dynamics of imperfect Batesian and Müllerian mimicries. Evolution 58:1294–1302. https://doi.org/10.1111/j.0014-3820.2004.tb01708.x
Mappes J, Marples N, Endler JA (2005) The complex business of survival by aposematism. Trends Ecol Evol 20:598–603. https://doi.org/10.1016/j.tree.2005.07.011
Marples NM, Roper TJ (1996) Effects of novel colour and smell on the response of naive chicks towards food and water. Anim Behav 51:1417–1424. https://doi.org/10.1006/anbe.1996.0145
Mazur MM, Beauchamp DA (2003) A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environ Biol Fishes 67:397–405. https://doi.org/10.1023/A:1025807711512
Monello RJ, Wright RG (2001) Predation by goldfish (Carassius auratus) on eggs and larvae of the eastern long-toed salamander (Ambystoma macrodactylum columbianum). J Herpetol 35:350–353. https://doi.org/10.2307/1566132
Nelson DWM, Crossland MR, Shine R (2011) Foraging responses of predators to novel toxic prey: effects of predator learning and relative prey abundance. Oikos 120:152–158. https://doi.org/10.1111/j.1600-0706.2010.18736.x
Neumeyer C (1992) Tetrachromatic color vision in goldfish: evidence from color mixture experiments. J Comp Physiol A 171:639–649. https://doi.org/10.1007/BF00194111
Neumeyer C, Mora-Ferrer C (2001) Color vision in goldfish and its neural basis. In: Backhaus W (ed) Neuronal Coding Of Perceptual Systems. Series on Biophysics and Biocybernetics. World Scientific, Berlin, pp 106–118
Nomura F, do Prado VHM, da Silva FR et al (2011) Are you experienced? Predator type and predator experience trade-offs in relation to tadpole mortality rates. J Zool 284:144–150. https://doi.org/10.1111/j.1469-7998.2011.00791.x
Paradise CJ, Stamp NE (1991) Prey recognition time of praying mantids (Dictyoptera: Mantidae) and consequent survivorship of unpalatable prey (Hemiptera: Lygaeidae). J Insect Behav 4:265–273. https://doi.org/10.1007/BF01048277
Peterson JA, Blaustein AR (1991) Unpalatability in anuran larvae as a defense against natural salamander predators. Ethol Ecol Evol 3:63–72. https://doi.org/10.1080/08927014.1991.9525389
Puurtinen M, Kaitala V (2006) Conditions for the spread of conspicuous warning signals: a numerical model with novel insights. Evolution 60:2246–2256. https://doi.org/10.1554/06-227.1
Richardson MJ, Whoriskey FG, Roy LH (1995) Turbidity generation and biological impacts of an exotic fish Carassius auratus, introduced into shallow seasonally anoxic ponds. J Fish Biol 47:576–585. https://doi.org/10.1111/j.1095-8649.1995.tb01924.x
Rojas B, Nokelainen O, Valkonen J (2017) Aposematism. In: Shackelford TK, Weekes-Shackelford VA (eds) Encyclopedia of evolutionary psychological science. Springer, pp 345–349
Rowe C, Guilford T (1996) Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature 383:520–522. https://doi.org/10.1038/383520a0
Rowland HM, Wiley E, Ruxton GD et al (2010) When more is less: the fitness consequences of predators attacking more unpalatable prey when more are presented. Biol Lett 6:732–735. https://doi.org/10.1098/rsbl.2010.0207
Rowland HM, Fulford AJT, Ruxton GD (2017) Predator learning differences affect the survival of chemically defended prey. Anim Behav 124:65–74. https://doi.org/10.1016/j.anbehav.2016.11.029
Ruxton GD, Sherratt TN, Speed MP (2004) Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford University Press, Oxford
Ruxton GD, Franks DW, Balogh ACV, Leimar O (2008) Evolutionary implications of the form of predator generalization for aposematic signals and mimicry in prey. Evolution 62:2913–2921. https://doi.org/10.1111/j.1558-5646.2008.00485.x
Savini D, Occhipinti-Ambrogi A, Marchini A et al (2010) The top 27 animal alien species introduced into Europe for aquaculture and related activities. J Appl Ichthyol 26:1–7. https://doi.org/10.1111/j.1439-0426.2010.01503.x
Schuler W, Roper TJ (1992) Responses to warning coloration in avian predators. Adv Study Behav 21:111–146. https://doi.org/10.1016/S0065-3454(08)60143-6
Sherratt TN (2002) The coevolution of warning signals. Proc R Soc B Biol Sci 269:741–746. https://doi.org/10.1098/rspb.2001.1944
Sillén-Tullberg B (1985) Higher survival of an aposematic than of a cryptic form of a distasteful bug. Oecologia 67:411–415. https://doi.org/10.1007/BF00384948
Sillén-Tullberg B, Wiklund C, Järvi T et al (1982) Aposematic coloration in adults and larvae of Lygaeus equestris and its bearing on Müllerian mimicry: an experimental study on predation on living bugs by the great tit Parus major. Oikos 39:131–136. https://doi.org/10.2307/3544476
Skelhorn J, Rowe C (2006) Avian predators taste-reject aposematic prey on the basis of their chemical defence. Biol Lett 2:348–350. https://doi.org/10.1098/rsbl.2006.0483
Skelhorn J, Halpin CG, Rowe C (2016) Learning about aposematic prey. Behav Ecol 27:955–964. https://doi.org/10.1093/beheco/arw009
Speed MP (2000) Warning signals, receiver psychology and predator memory. Anim Behav 60:269–278. https://doi.org/10.1006/anbe.2000.1430
Stevens M, Ruxton GD (2012) Linking the evolution and form of warning coloration in nature. Proc R Soc B Biol Sci 279:417–426. https://doi.org/10.1098/rspb.2011.1932
Svádová KH, Exnerová A, Štys P (2014) Gregariousness as a defence strategy of moderately defended prey: experiments with Pyrrhocoris apterus and avian predators. Behaviour 151:1617–1640. https://doi.org/10.1163/1568539X-00003208
Üveges B, Fera G, Móricz ÁM et al (2017) Age-and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol Biol 17:1–10. https://doi.org/10.1186/s12862-017-0956-5
Vogel JL, Beauchamp DA (1999) Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Can J Fish Aquat Sci 56:1293–1297. https://doi.org/10.1139/cjfas-56-7-1293
Voris HK, Bacon JP (1966) Differential predation on tadpoles. Copeia 1966:594. https://doi.org/10.2307/1441096
Waldman B, Adler K (1979) Toad tadpoles associate preferentially with siblings. Nature 282:611–613. https://doi.org/10.1038/282611a0
Wang LY, Huang WS, Tang HC et al (2018) Too hard to swallow: a secret secondary defence of an aposematic insect. J Exp Biol 221:jeb172486. https://doi.org/10.1242/jeb.172486
Wells KD (2013) The Ecology and behavior of amphibians. University of Chicago Press
Wiklund C, Järvi T (1982) Survival of distasteful insects after being attacked by naive birds: a reappraisal of the theory of aposematic coloration evolving through individual selection. Evolution 36:998–1002. https://doi.org/10.1111/j.1558-5646.1982.tb05468.x
Winandy L, Denoël M (2013) Introduced goldfish affect amphibians through inhibition of sexual behaviour in risky habitats: an experimental approach. PLoS One 8:e82736. https://doi.org/10.1371/journal.pone.0082736
Winandy L, Denoël M (2015) The aggressive personality of an introduced fish affects foraging behavior in a polymorphic newt. Behav Ecol 26:1528–1536. https://doi.org/10.1093/beheco/arv101
Yamazaki Y, Pagani-Núñez E, Sota T, Barnett CRA (2020) The truth is in the detail: predators attack aposematic prey with less aggression than other prey types. Biol J Linn Soc 131:332–343. https://doi.org/10.1093/biolinnean/blaa119
Acknowledgements
Matthew Noakes kindly commented on the early version of the manuscript.
Funding
This study was supported by the funding from the Faculty of Veterinary Medicine and Animal Science (No. 506.511.09.00), Poznań University of Life Sciences.
Author information
Authors and Affiliations
Contributions
MK: conceptualization, data curation, funding acquisition, investigation, project administration, writing and editing; JMK: investigation and resources; KK: formal analysis, investigation, and writing and editing; KB: formal analysis, investigation; JK: formal analysis, investigation; JK: conceptualization, formal analysis, investigation, writing and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Ethics approval
The use of protected species was approved by the Regional Directorate for Environmental Protection (Permit No. WPN-II.6401.36.2016.AS.2). After the test, all live and healthy animals were released at the capture site following the permission granted to us. We followed all applicable institutional and national guidelines for the care and use of animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaczmarski, M., Kaczmarek, J.M., Kowalski, K. et al. Increasingly cautious sampling, not the black colouration of unpalatable prey, is used by fish in avoidance learning. Anim Cogn 26, 1705–1711 (2023). https://doi.org/10.1007/s10071-023-01815-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-023-01815-9