Abstract
Living in a herd has multiple advantages for social species and is a primary survival strategy for prey. The presence of conspecifics, identified as a social buffer, may mitigate the individual stress response. Social isolation is, therefore, particularly stressful for horses, which are gregarious animals. However, they are not equally vulnerable to separation from the group. We tested whether more and less socially dependent horses and independent individuals would differ in their responses to novel and sudden sounds occurring in two contexts: non-social and social motivation. Twenty warmblood horses were first exposed to two social tests: to evaluate the level of social dependence (rate of restless behaviour; social isolation) and the quantity and the quality of interactions in which they were involved (stay on a paddock). Two fear audio tests were then performed to compare the responses to sudden sounds while feeding (non-social motivation; control trial) and while moving towards the herd (social motivation; experimental trial). Socially dependent horses showed more pronounced avoidance behaviour and needed much more time to resume feeding during the control trial. Hence, dependent individuals appeared to be more fearful. However, during an experimental trial, horses of both groups tended to ignore the sound or paid only limited attention to the stimulus, continuing to move forward towards their conspecifics. Thus, social motivation may mitigate fear caused by a frightening stimulus and make fearful and dependent horses more prone to face a potentially stressful event. This finding should be taken into account in horse training and management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Horses’ reactivity to different stressors and environmental stimuli is modified by the animal’s motivation, fearfulness or curiosity (Kozak et al. 2018). The variety of these responses may stem from differences in coping styles or temperamental traits (Visser et al. 2002) and has major implications in animal husbandry (Rothmann et al. 2014). Responding to suddenness and novelty may compromise both the safety of the handler and the welfare of a horse (Acton et al. 2020; Vidament et al. 2021). It is, therefore, crucial to be aware of the possible reactions of the horses (Corgan et al. 2021). Temperament is defined as any characteristic of an individual that appears to be stable over both time and situation (Roberts et al. 2016). Fearfulness and gregariousness, i.e. reactivity to social isolation from conspecifics (Lansade et al. 2008b), are among the most well-known and particularly interesting dimensions of equine temperament (Lansade et al. 2008a, 2017; Górecka-Bruzda et al. 2022). Indeed, these traits relate directly to the evolution of the horse, which is a highly social prey animal that lives in a herd and remains in constant readiness to detect and avoid the threat (Marliani et al. 2021).
A stable and organised social structure, manifested by dominance and submissive interactions, enables horses to avoid the deleterious energy and health costs that would be incurred in aggressive encounters (Narciso et al. 2021). The group members help to improve foraging strategies, provides learning opportunities (Krueger and Heinze 2008; Krueger et al. 2014; Mendonça et al. 2021) and facilitates localising and avoiding a potential threat (Hothersall and Casey 2012). For social species, living in a herd is the primary antipredator defence mechanism (Apfelbach et al. 2005). Although predation pressure is relaxed in domestic horses (Janczarek et al. 2021), they experience multiple anthropogenic stressors (Squibb et al. 2018). According to the risk-disturbance hypothesis, various sounds, objects and events may trigger a response analogous to the presence of a predator (Frid and Dill 2002). In this context, social support from other members of the herd consists of social buffering that mitigates individual stress levels (Hartmann et al. 2012).
When the alternative is no possibility of social interaction, horses are highly motivated to make any kind of physical contact (full, head or muzzle) with other individuals (Søndergaard et al. 2011). They strengthen relationships through affiliative approaches, mutual grooming or keeping proximity (Wolter et al. 2018). However, if direct contact is not possible, they at least strive for indirect interactions (Yarnell et al. 2015). For horses, social isolation is a particularly strong stressor that negatively affects their behavioural and physiological reactivity (Kay and Hall 2009; Żelazna and Jezierski 2018). When returning to the group after a previous restriction of social opportunities, horses may manifest a rebound effect, which refers to increased intensity of interactions with conspecifics (Christensen et al. 2002a). Stress elicited by social isolation may even moderate mild somatic pain (Reid et al. 2017). However, horses do not respond to the separation from the group with the same strength (Wolff et al. 1997). Socially dependent individuals have difficulty functioning when out of the herd. In contrast, independent animals are not excessively bonded to other horses or humans and are self-reliant when left away from the herd (Burattini et al. 2020). In the previous study (Janicka et al. 2022a), we showed that the motivation for social interaction rapidly decreased the potential of a self-applied acoustic stimulus in creating virtual barriers. Only 20% of horses turned back or stopped after the playback of acute alarming sound (sudden, unexpected) when the second horse was present in the excluded zone. In turn, when food reward was offered in that zone, the virtual barrier was 80% effective and made the horses respond with flight, going away or stopping.
Here, we assessed if horses characterised by different levels of social dependence would differ in their fear reaction to the sudden sounds occurring in two different contexts: social (while moving towards the herd) and non-social (while consuming attractive food). Given the significance of the social nature of horses, we hypothesised that socially dependent and independent individuals may show different fear responses in the non-social, but similar and weak responses in the social context, which would highlight the importance of social motivation in reducing the effect of frightening stimulus.
Materials and methods
Ethical statement
All experimental procedures were carried out in accordance with the European directive 2010/63/EU and Polish laws—the Act of January 23, 2021—Law on the organization of breeding and reproduction of farm animals (Journal of Laws 2021, Item 36) and were approved by the Local Ethics Review Committee for Animal Experiments (no 58/2022), Lublin, Poland.
Animals and living conditions
The study involved 20 adult warmblood horses (aged 6–16 years; 10.6 ± 3.6 years), comprising ten mares and ten geldings. The horses knew each other as they had been housed in the same equestrian centre for at least two years. They were released to an extensive pasture or occasionally to several paddocks for about six hours a day, where they could engage in social interaction. All of the subjects were familiar with the paddocks and pastures used in the study. Thus, habituation to the environment was not necessary.
The subjects were kept in individual box stalls bedded with wheat straw and equipped with a manger, hay feeder, salt lick and an automatic waterer. They were fed three times a day with a mixture of oats and bran and had unlimited access to hay. The horses worked under the saddle for a maximum of two hours a day, six days a week. The subjects were under permanent veterinary control (periodic physical examination, assessment during the movement, anamnesis, monitoring heart rate) and were regularly observed by an experienced caretaker during daily handling. Additionally, they were examined one week before and after the study (the above procedures). No physical or behavioural disorders were found before or after the study.
The tests were carried out on calm, windless days (< 0.3 m/s) during the spring period of 2022 and 2023. The location of the testing area reduced unnecessary stimuli which could interfere with the perception of the sound stimulus and affect horse’s reactions, including auditory stimuli, movement of vehicles, pedestrians, or other horses. No sudden and loud noises (e.g. dog barking, tractor noise, or other equipment) interrupted the testing procedures.
Two adult female experimenters handled the horses during the study. They were known to the horses, as they were also their caretakers and looked after them five days a week.
Social tests
Prior to the main part of the experiment (Sound tests), the horses were subjected to two different social tests to assess the level of social dependence and engagement in social interactions. The tests were conducted on separate, consecutive days.
Social dependence test–arena test
Dependence on the other members of the social group was assessed during the herd (social) dependence test. The test consisted of separation from a herd that stayed on the pasture about 300 m from a tested subject. Horses were individually released to a small paddock (400 m2) and were observed for a subsequent five minutes using the focal animal sample method (Altmann 1974). This time appeared to be sufficient because individual differences in reactions to an unusual situation were shown to be the strongest within the first few minutes (Wolff et al. 1997).
Behaviours that were assessed during the test were chosen based on the ethogram used in the arena test by Seaman et al. (2002), but some modifications were implemented. The most frequent behavioural variables indicating negative emotional arousal during isolation were vocalisation, defecation, locomotion and vigilance (Lansade et al. 2008b). Thus, certain behaviours assessed by Seaman et al. (2002) (standing, investigation, tail posture, paw, urination) were waived and the traits: ‘canter’ and ‘snoring’ (instead of snorts produced in positive context (Laurijs et al. 2021)) were added to the ethogram used in the current study. Behaviours assessed as frequency (whinny, snoring and defecation) were primarily recorded. However, since in the current study they were displayed only rarely by a few horses, they were not included in the Table 1 and were not considered when dividing horses into groups with different levels of social dependence.
After conducting and analysing the data from the social dependence test, horses were divided into three groups of social dependence. The level of social dependence was determined on the self-established percentage time of displaying restless behaviours based on variation of horses’ responses:
-
independent (I): < 15% (n = 9) of the observation time was accounted for by restless behaviour–horses able to function without the social support of conspecifics (Stachurska et al. 2021),
-
moderately dependent (MD): 15–50% (n = 5),
-
dependent (D): > 50% (n = 6)–horses not self-reliant, reactive to social isolation from conspecifics (Burattini et al. 2020).
Social interaction test–introduction to a paddock
To determine the engagement in social interactions, the horses were observed just after being released into a large paddock (5000 m2) in the morning (8.00 a. m.). The procedure was repeated on three consecutive days, with a single observation session lasting ten minutes. This observation time was taken from the study of Seaman et al. (2002), who assessed the dominant and submissive behavioural responses of horses after reintroduction to the group.
Affiliative and agonistic interactions (McDonnell 2003) (Table 2) in which each focal animal was involved were recorded every 30 s using the one-zero sampling method (Altmann 1974). The total number of affiliative, aggressive/threatening and submissive behaviours exhibited by each horse was then calculated. The measurement of spatial proximity (horses standing with body contact or within two horse-lengths), commonly used to assess social bonds (Wolter et al. 2018), was not taken into account because of the short-term character of the social test, whereas horses show a mean latency of changing the spatial distribution of group members every 8 min (Christensen et al. 2002b).
Sound tests
Sound tests were the startling fearfulness tests, assessing horses’ reactions to a sudden auditory stimulus (Seaman et al. 2002). However, these tests were conducted in two different contexts: without (a control trial) and with (an experimental trial) a social motivation. Each sound test was carried out twice (two stages) with an interval of about a year (to reduce the likelihood of habituation to the sounds). Two different sounds were used during these tests: sound A (squeal of a pig, produced during distress (Çavuşoğlu et al. 2020)) and sound B (futuristic characteristics; similarity to radio and TV interference, crackling). The sounds were selected based on the results of our previous studies: comparing reaction to 40 sounds of different origin (Janicka et al. 2022a), assessing the possibility to use sound stimulus in creating virtual barrier (Janicka et al. 2022b) and comparing reactions to 40 known and four novel sounds (unpublished data). Sounds that were chosen elicited similar and strongest avoidance behaviour. Both sounds were unknown to the study horses. In the first stage of the study (stage 1; 2022 year), sound A was played during the control trial and sound B during the experimental trial. The reverse order was then used in the second stage (stage 2; 2023 year). In addition, 10 randomly selected horses were first subjected to the experimental and then to the control trial and for the remaining 10 horses, the order of procedures was reversed. The experimental trial was conducted in the morning (7.30–10.30) when horses were normally released to the extensive pasture to induce the motivation to move forward and to join the herd. In turn, the control trial was carried out in the afternoon/evening hours (15.30–19.30) when horses were brought back to the stable and were used to stay in boxes or paddocks near the stable (reduction of social motivation). The interval between two trials was at least six days (6–8 days; differences caused by poor weather conditions: rain or wind > 0.3 m/s).
Non-social motivation sound test (NMST)–a control trial
The horses were individually subjected to a controlled assessment of their fear response to a new and unexpected sound while eating (non-social motivation sound test; NMST). The testing arena (140 × 30 m) was designated with tape for an electric fence on one of the known paddocks. It was located next to another paddock, providing proximity (approximately 50 m) to conspecifics during the test to avoid the impact of social isolation stress and lack of concentration on the food provided. The three horses in the neighbouring paddock (the same animals throughout the experiment, visible to study subjects) were habituated to the test sound a few days before the study.
A plastic container for the later provision of attractive food was placed at the front of the testing arena. A loudspeaker (JBL Charge 4, rated power of 30W) was positioned 1 m in front of the container, allowing the horse a long escape distance after the sound playback, if necessary. Prior to the test, horses were taught to feed from the container.
Each horse was released into a testing arena 15 min before the start of the test to habituate to the experimental setting. The first experimenter (E1) then brought the horse to the start point (3 m from the container) and waited for the test to begin. At this time, the second experimenter (E2) put chopped carrots inside the container. The horse was unleashed (by E1) and could start feeding, and both experimenters left the arena. After 30 s, E2 played a sudden sound (20 s, 40 dB; first stage–sound A, second stage–sound B) via Bluetooth by a Samsung Galaxy A02s device (Samsung Electronics Co., Ltd., South Korea). As the maximum range of Bluetooth was approximately 15 m, experimenters had to stay at this distance during the test in order to play the recording at the right moment. However, they stood calmly, did not disrupt the test and the horses were used to their presence (they were also their caretakers).
Latency to respond to the stimulus was proved to be the objective and easy measure of horses’ responsiveness in fear tests (Górecka-Bruzda et al. 2011). Therefore, latency to resume feeding (s) was measured in the current study. If a horse did not approach the food within 300 s, it was given 301 s (Górecka-Bruzda et al. 2011). Additionally, a 5–point behavioural scale describing horses’ reactions after sound playback was developed. Receiving 5 points indicated the highest level of fear (Table 3).
Social motivation sound test (SMST)–an experimental test
The social motivation sound test (SMST) was a modified version of the sound tunnel test developed in the previous study (Janicka et al. 2022a) to assess the potential of the sounds in creating electric-free virtual fences. During the test, the horses moved through the same corridor (55 m length, 5 m width, designated with tape for an electric fence) leading towards a known pasture, where the other members of the herd (except for individuals included in the study) were staying. The start–end of the corridor was closed after the introduction of a horse to a start zone (first 5 m), whereas the finish-end was permanently open, allowing the animal to leave it after each run. The experimental paddock, at which the corridor was designated, was separated from the pasture by metal railings. There was a loudspeaker placed outside the corridor at the finish line. The day before the SMST, horses were brought into the experimental paddock for two hours to familiarise themselves with the corridor.
SMST was conducted in the morning hours on three consecutive days (three trials). During each trial, horses went through the corridor at least three times: (1) being led by the experimenter (E1), (2) freely, without the experimenter (E1), and (3) freely, but with playing a sudden sound by E2 (20 s, 100 dB; first stage–sound B, second stage–sound A) when the individual reached a distance of 10 m from the finish line. The initial two trials evaluated the horse's willingness to walk towards the pasture and other horses located approximately 150 m away. If the horse exhibited this behaviour, a third trial was conducted to observe its reactions. Before each trial, the study subject was held on the line in a start zone by E1. The second experimenter (E2) waited in a distance of 15 m from the loudspeaker to play the sound at the right moment after the horse was released by E1.
We showed that the motivation for social interaction rapidly decreased the audio virtual fencing effect (Janicka et al. 2022a). Thus, we focused on subtle differences in reaction to the sound between horses with different levels of social characteristics. The sound barrier effect, completion of the SMST and behaviour after completion of the test were recorded (Table 4).
Statistical analyses
The statistical analysis was performed with the SAS 9.4 software (version 9.4, SAS Institute Inc., Cary, NC, USA). Since the data did not conform to a normal distribution, as determined by the Kolmogorov–Smirnov and Shapiro–Wilk tests (p < 0.05), non-parametric tests were applied. Correlations between the results of behavioural tests were checked with + Spearman's rank correlation. Kruskal–Wallis test was used to evaluate if social dependence affected the horses’ responses to the sounds and engagement in affiliative and dominant-submissive interactions. Dwass-Steel-Critchlow-Fligner (DSCF) multiple comparisons were then performed to check for the significance of differences between the groups. Additionally, the differences between mares (n = 10) and geldings (n = 10) and between younger (n = 10; 6 – 10 years) and older horses (n = 10; 11–16 years) during the tests (Mann–Whitney U test), as well as between two stages of the study (Wilcoxon test) were checked. Even though the significance of results was calculated via non-parametric tests, for better clarity and readability for readers, the results were presented in tables as mean value ± standard error of the mean (SE). The minimum level of significance for all tests used was accepted at p < 0.05.
Results
Stage of the study differences
No significant differences were found between the results of sound tests in two stages of the study (the revised order of sounds used in the control and the experimental trial) (p > 0.05) (Table 5). Horses responded similarly, no matter which sound (A–squeal of a pig, or B–futuristic sound) was used as control or experimental. For this reason, further comparisons between the reaction in two stages were waived.
Dependence on the social group and need for social interactions
Most of the horses (n = 11) showed more (n = 6; dependent) or lesser (n = 5; moderately dependent) signs of anxiety when they were physically isolated from the group (Table 6). Of all the other horses, those categorised as dependent spent the most time demonstrating a sustained walk (DSCF = 3.8730, p = 0.0170; DSCF = 5.0767, p = 0.0010; in comparison to moderately dependent and independent, respectively). The time of vigilance was similar in the dependent and moderately dependent groups (DSCF = 0.2582, p = 0.9818) and definitely longer than in independent horses (DSCF = 4.1779, p = 0.0088; DSCF = 4.2567, p = 0.0074, respectively). Canter rarely occurred and did not differ significantly between the groups (p > 0.05). Dependent horses trotted (DSCF = 3.9485, p = 0.0145) more than independent horses but not moderately dependent individuals (DSCF = 2.7749, p = 0.1217; DSCF = 2.9863, p = 0.0875, respectively).
Although horses responded differently to social isolation, the quantity and the quality of interactions during their stay on a paddock (social interaction test) were similar between the groups (p > 0.05). The horses usually displayed affiliative interactions, whereas dominance and submissive behaviours were rarely observed. For this reason, agonistic behaviours were not included in further analysis.
Reactions to sounds
All the horses (n = 20) moved willingly towards the pasture in SMST in the first two trials and were subjected to the third trial (the proper test), in which their reactions were assessed.
Horses’ responses to a sudden sound in NMST varied, but they generally interrupted feeding for some time (stage 1; n = 17, stage 2; n = 18) and showed movement in the opposite direction (stage 1; n = 13, stage 2; n = 16). In contrast, they rarely stopped forward movement (stage 1; n = 4, stage 2; n = 5) and turned back towards the start zone (stage 1; n = 2, stage 2; n = 1) in SMST. Their reactions were usually limited to slowing down after hearing a sound while moving towards the other herd members.
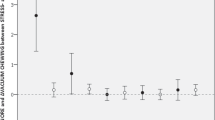
Dependent horses were more fearful during NMST than independent horses (Table 7). They needed much more time to resume feeding after the sound was played (DSCF = 6.0376, p < 0.0001) and had higher scores on the behavioural scale, indicating the strength of the fear response (DSCF = 6.3439, p < 0.0001). In spite of this, no differences were found between the groups during the SMST (p > 0.05). The sound barrier effect was poor and usually limited to slowing down or stopping for a while, and eventually, most of the horses finished the test.
There were no correlations between the horses’ reactions in two different sound tests, except for the first reaction after the sound playback (behavioural scale) and latency to refeed in NMST and barrier effect in SMST (Table 8). In the case of the horses that gained more points on the behavioural scale (stronger fear response) and needed more time to resume feeding, the sound barrier effect was greater (Spearman's rank correlation; rs = 0.350, p < 0.0001 and rs = 0.325, p = 0.0003, respectively). However, these correlations were weak.
Both refeeding latency (rs = 0.354, p < 0.0001) and behavioural scale scoring (rs = 0.397, p < 0.0001) were weakly correlated with the time spent on restless behaviour in the herd dependence test. Simultaneously, restlessness (indicating the level of herd dependence) and the responses during SMST were not correlated (p > 0.05).
There was no correlation between the frequency of affiliative interactions and social dependence (restlessness) (rs = 0.172, p = 0.1898). However, in the case of the horses that interacted more, the sound barrier effect was stronger (more points in the behavioural scale) (rs = 0.416, p < 0.0001) and the horses finished the test with a lower probability (rs = -0.349, p < 0.0001). They also received more points on the behavioural scale during NMST (rs = 0.491, p < 0.0001) and had longer refeeding latencies (rs = 0.315, p = 0.0005).
Sex and age differences
Mares were more restless when separated from the group during the herd dependence test (Mann–Whitney U test; U = 256.50, p = 0.0037), needed more time to resume feeding after sound playback in NMST (U = 1156.50, p = 0.0007) and responded to this sound more violently (they had a higher score on the behavioural scale) (U = 1260.00, p = 0.0046) than geldings (Table 9). Showing affiliative interactions was common and comparable (U = 427.50, p = 0.7412). Although mares showed submissive behaviour more often (U = 297.00, p = 0.0234), agonistic interactions were rarely presented. No differences between mares and geldings were found in response to the sound during SMST (p > 0.05). The sound barrier effect (stopping/limiting further movement) was weak, and both mares and geldings finished the test with a similar frequency. The only difference concerned behaviour after completion of the SMST. Mares were more agitated while waiting to be reunited with the herd (U = 1030.00, p < 0.0001).
There were no differences between the responses of younger and older horses during the herd dependence test, NMST and SMST (p > 0.05). Only during the social interaction test younger individuals showed significantly more affiliative interactions (U = 1134.00, p = 0.0005).
Discussion
Horses evolved as prey species for which living in a herd is a survival strategy (Marliani et al. 2021). Thus, isolation from conspecific is a particularly strong stressor for horses (Kay and Hall 2009). However, similarly to Wolff et al. (1997), Lansade et al. (2008b) and Górecka-Bruzda et al. (2022), we showed that they were not equally vulnerable to separation from the herd. The differences in isolation anxiety were mainly displayed by various engagement in sustained walking, vigilance and trotting, whereas neighing, snorting and defecation were infrequent. In turn, Lansade et al. (2008b) point to the frequency of neighing as the most interesting behavioural parameter, which was well correlated with other parameters measured in the same isolation tests, such as defecation, locomotion and vigilance. The differences in these observations may be due to the individual characteristics of the horses. Also some possibility of auditory contact between the study subjects and the herd (staying in a distance of 300 m) can not be entirely excluded, which is a limitation of our study. Besides the existence of a trait ‘social dependence’, the isolation test also revealed the importance of the factor ‘sex’ on gregariousness. We noticed that, compared to geldings, mares were more restless when separated from the herd. This observation is in agreement with the study of Górecka-Bruzda et al. (2022), who showed that mares were more socially dependent than geldings. The current study further showed that social dependence is not manifested by engagement in social interactions. After the introduction to a large paddock during the social interaction test, the horses presented a wide range of affiliative interactions, including mutual grooming, following, approach and motor playing. The frequency of affiliative relationships did not vary between horses of different levels of gregariousness, or between mares and geldings. The similar engagement in interactions of horses with different social characteristics stresses the importance of social contact with conspecifics. Additionally, the fact that horses with different levels of gregariousness made a similar number of social interactions indicates that correlation between engagement in affiliative interactions and higher agitation after sound playback both in a non-social and social motivation context were due to greater fearfulness rather than social dependence. We further confirmed that social dependence does not result from the need for direct interactions but rather from the very need to stay close to the herd and is best observed during social isolation (Le Scolan et al. 1997; Wolff et al. 1997; Seaman et al. 2002; Lansade et al. 2008b; Lansade and Simon 2010). However, we did not consider spatial proximity between the horses in the social interaction test, which, as one of the social bond measurements, could show correlations with social dependence. It would be worthwhile to include this trait in future research. In the current study agonistic interactions were rare and limited exclusively to threatening, so it was not possible to compare such behaviour in dependent and independent individuals. Similarly, Kimura (1998) noticed that during spring season, thus, when breeding season starts, dominance–submission interactions were not frequent between free-ranging mares. We also observed horses in spring, but in the case of our study the results could stem from a short period of observation. Furthermore, unlike Kimura’s (1998) study, our experiment was not conducted at different seasons of the year. The limitation of social interaction test after introduction to a paddock (even if simple and easy to repeat) in assessing the full range of equine social behaviour should be taken into account in further studies.
As we predicted, horses of different levels of social dependence varied in their responses to the sudden sound during the test in a non-social (but not a social) motivation context when they moved towards a herd on a pasture. Horses classified as dependent were more fearful compared to independent individuals in a non-social motivation context. They responded to the sound more violently (jumped back and walked/run away more often) and needed significantly more time to resume feeding than self-reliant animals. Briard et al. (2015) also noted that fearless horses were simultaneously less gregarious. In turn, Villalba et al. (2009) showed that sheep cautious in accepting new food were more reactive during social isolation. Indeed, in many species, these traits have been used to characterise ‘bold’ and ‘shy’ behavioural types (Briard et al. 2015). We found no correlations between the rate of restlessness during separation from the herd and the results of the social motivation sound test. Horses’ reactions were usually limited to slowing down after hearing the sound or eventually to stopping for a while. Even when hesitating, they decided to move forward to stay closer to the herd. Thus, the motivation to join the group was too strong for an unexpected, novel sound to stop both independent and dependent horses from further movement. Jouven et al. (2012) showed that some of the sheep, previously trained not to exceed the virtual fence, followed their conspecifics regardless of the electric shocks they received. Since, in our study, dependent and moderately dependent individuals showed a greater level of fear during a control sound test and then reacted similarly to independent horses in the social motivation sound test, we may conclude that the strong need to stay in proximity to members of the group mitigates fear caused by a frightening stimulus. As reported by Burattini et al. (2020), boldness and independence are two important behavioural traits that influence horses' fearfulness, assertiveness and sociability. Socially dependent horses are not at ease when left alone, away from the herd. Instead, they rely more on their conspecifics (Burattini et al. 2020). However, it should be noted that sound tests used in our study differed in the nature of the motivational conflict. In the non-social motivation test horses had to choose between staying and eating attractive food or fleeing (stay-avoidance conflict), whereas in social motivation test they had to decide whether to continue moving forward towards the herd or to stop/turn back to avoid the frightening stimulus (moving forward-avoidance conflict). Also, unlike in the test with food, the horses had no reason to stay near the loudspeaker once they had passed it. The mentioned differences between two tests may be the reason for limited correlations between horses’ responses to sound playback in different motivational conflicts. Lansade et al. (2008c) showed that within each sense, the greater the horses’ response to one stimulus, the greater their response to the other. However, as it was shown in our study, it is important to regard the context of presenting a testing stimulus during behavioural observations.
In the current study, also mares, that were shown to be more socially dependent than geldings, were definitely more anxious during a control sound test but reacted the same as the geldings during the sound test in the social motivation context. Thus, willingness to be reunited with conspecifics reduced the effect of a frightening stimulus. The only difference concerned behaviour after completion of a test, when the horses were waiting to join the herd. Again, the mares were more agitated, similar to the social isolation test (social dependence test). Lansade et al. (2022) noted that weaned horses still preferred their dam as well as other mares from their natal group after five months of separation, but this tendency was stronger in fillies than in colts. A stronger need to stay in a group in the case of mares may be due to the social structure of the species. In the wild, horses lived in groups comprising mainly females, and this is probably why social support is particularly important for them (Górecka-Bruzda et al. 2022). However, it should be noted that the geldings may have reacted differently than it would be seen under natural conditions in stallions.
The results of the current study may have practical implications. Horse owners should be aware of the existence of social dependence in horses and its correlations with fearfulness. However, similarly to Christensen et al. (2008) and Ricci‑Bonot et al. (2021), we confirmed the role of conspecifics as a social buffer and the importance of social motivation to confront a stressor. For example, in the study of Dai et al. (2019), horses were taught loading into a trailer in groups, which allowed their gregarious nature to be used in the learning process. We conclude that this factor should be considered in horse management when handling or schooling animals.
Conclusions
Horses are highly motivated to stay close to their conspecifics, but they differ in their responses to social isolation. Socially dependent horses are not at ease being left alone outside the herd, in contrast to those more independent. However, while on the paddock, they engage in social interactions in a similar way. Dependent individuals are generally more fearful than independent animals. However, when facing sudden, novel audio stimulus in a social motivation context (motivation to join a herd), they react similarly to more self-reliant horses. Thus, social motivation may mitigate fear caused by a frightening event in horses. This finding should be taken into account in horse training and management.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Acton AS, Gaw CE, Chounthirath T, Smith GA (2020) Nonfatal horse-related injuries treated in emergency departments in the United States, 1990–2017. Am J Emerg Med 38:1062–1068. https://doi.org/10.1016/j.ajem.2019.158366
Altmann J (1974) Observational study of behavior–Sampling methods. Behaviour 49:227–267
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144. https://doi.org/10.1016/j.neubiorev.2005.05.005
Briard L, Dorn C, Petit O (2015) Personality and affinities play a key role in the organisation of collective movements in a group of domestic horses. Ethology 121:888–902. https://doi.org/10.1111/eth.12402
Burattini B, Fenner K, Anzulewicz A, Romness N, McKenzie J, Wilson B, McGreevy P (2020) Age-related changes in the behaviour of domestic horses as reported by owners. Animals 10:2321. https://doi.org/10.3390/ani10122321
Çavuşoğlu E, Rault J-L, Gates R, Lay DC Jr (2020) Behavioral response of weaned pigs during gas euthanasia with CO2, CO2 with Butorphanol, or Nitrous Oxide. Animals 10:787. https://doi.org/10.3390/ani10050787
Christensen JW, Ladewig J, Søndergaard E, Malmkvist J (2002a) Effects of individual versus group stabling on social behaviour in domestic stallions. Appl Anim Behav Sci 75:233–248. https://doi.org/10.1016/S0168-1591(01)00196-4
Christensen JW, Zharkikh T, Ladewig J, Yasinetskaya N (2002b) Social behaviour in stallion groups (Equus przewalskii and Equus caballus) kept under natural and domestic conditions. Appl Anim Behav Sci 76:11–20. https://doi.org/10.1016/S0168-1591(01)00208-8
Christensen JW, Malmkvist J, Nielsen BL, Keeling LJ (2008) Effects of calm companion on fear reactions in naïve test horses. Equine Vet J 40:46–50. https://doi.org/10.2746/042516408X245171
Corgan ME, Grandin T, Matlock S (2021) Evaluating the reaction to a complex rotated object in the american quarter Horse (Equus caballus). Animals 11:1383. https://doi.org/10.3390/ani11051383
Dai F, Costa AD, Bonfanti L, Caucci C, Di Martino G, Lucarelli R, Padalino B, Minero M (2019) Positive reinforcement-based training for self-loading of meat horses reduces loading time and stress-related behavior. Front Vet Sci 6:350. https://doi.org/10.3389/fvets.2019.00350
Frid A, Dill L (2002) Human–caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:11. https://doi.org/10.5751/ES-00404-060111
Górecka-Bruzda A, Jastrzębska E, Sosnowska Z, Jaworski Z, Jezierski T, Chruszczewski MH (2011) Reactivity to humans and fearfulness tests: field validation in Polish. Appl Anim Behav Sci 133:207–215. https://doi.org/10.1016/j.applanim.2011.05.011
Górecka-Bruzda A, Jastrzębska E, Drewka M, Nadolna Z, Becker K, Lansade L (2022) Female Horses are more socially dependent than geldings kept in riding clubs. Appl Anim Behav Sci 254:105714. https://doi.org/10.1016/j.applanim.2022.105714
Hartmann E, Søndergaard E, Keelinga LJ (2012) Keeping horses in groups: a review. Appl Anim Behav Sci 136:77–87. https://doi.org/10.1016/j.applanim.2011.10.004
Hothersall B, Casey R (2012) Undesired behaviour in horses: a review of their development, prevention, management and association with welfare. Equine Vet Educ 24:479–485. https://doi.org/10.1111/j.2042-3292.2011.00296.x
Janczarek I, Stachurska A, Kędzierski W, Wnuk-Pawlak E, Wilk I, Zyglewska K, Paszkowska A, Ryżak M, Wiśniewska A (2021) Heart rate variability in Konik and purebred Arabian horses in response to different predator vocalisations. Animal 15:100045. https://doi.org/10.1016/j.animal.2020.100045
Janicka W, Wilk I, Próchniak T, Janczarek I (2022a) Can sound alone act as a virtual barrier for horses? A Preliminary Study. Animals 12:3151. https://doi.org/10.3390/ani12223151
Janicka W, Wilk I, Ryżak M (2022) Horses’ perception of a threat posed by sounds of different origin. Med Weter 78:401–413. https://doi.org/10.21521/mw.6672
Jouven M, Leroy H, Ickowicz A, Lapeyronie P (2012) Can virtual fences be used to control grazing sheep? Rangel J 34:111–123. https://doi.org/10.1071/RJ11044
Kay R, Hall C (2009) The use of a mirror reduces isolation stress in horses being transported by trailer. Appl Anim Behav Sci 116:237243. https://doi.org/10.1016/j.applanim.2008.08.013
Kimura R (1998) Mutual grooming and preferred associate relationships in a band of free-ranging horses. Appl Anim Behav Sci 59:265–276. https://doi.org/10.1016/S0168-1591(97)00129-9
Kozak A, Zięba G, Tietze M, Rozempolska-Rucińska I (2018) Consistency of emotional reactivity assessment results obtained in different behavioural tests. Appl Anim Behav Sci 205:54–60. https://doi.org/10.1016/j.applanim.2018.05.013
Krueger K, Heinze J (2008) Horse sense: social status of horses (Equus caballus) affects their likelihood of copying other horses` behavior. Anim Cogn 11:431–439. https://doi.org/10.1007/s10071-007-0133-0
Krueger K, Farmer K, Heinze J (2014) The effects of age, rank and neophobia on social learning in horses. Anim Cogn 17:645–655. https://doi.org/10.1007/s10071-013-0696-x
Lansade L, Simon F (2010) Horses’ learning performances are under the influence of several temperamental dimensions. Appl Anim Behav Sci 125:30–37. https://doi.org/10.1016/j.applanim.2010.02.010
Lansade L, Bouissou M-F, Erhard HW (2008a) Fearfulness in horses: a temperament trait stable across time and situations. Appl Anim Behav Sci 115:182–200. https://doi.org/10.1016/j.applanim.2008.06.011
Lansade L, Bouissou M-F, Erhard HW (2008b) Reactivity to isolation and association with conspecifics: a temperament trait stable across time and situations. Appl Anim Behav Sci 109:355–373. https://doi.org/10.1016/j.applanim.2007.03.003
Lansade L, Pichard G, Leconte M (2008c) Sensory sensitivities: Components of a horse’s temperament dimension. Appl Anim Behav Sci 114:534–553. https://doi.org/10.1016/j.applanim.2008.02.012
Lansade L, Marchand AR, Coutureau E, Ballé C, Polli F, Calandreau L (2017) Personality and predisposition to form habit behaviours during instrumental conditioning in horses (Equus caballus). PLoS One 12:e0171010. https://doi.org/10.1371/journal.pone.0171010
Lansade L, Lévy F, Parias C, Reigner F, Górecka-Bruzda A (2022) Weaned horses, especially females, still prefer their dam after five months of separation. Animal 16:100636. https://doi.org/10.1016/j.animal.2022.100636
Laurijs KA, Briefer EF, Reimert I, Webb LE (2021) Vocalisations in farm animals: a step towards positive welfare assessment. Appl Anim Behav Sci 236:105264. https://doi.org/10.1016/j.applanim.2021.105264
Le Scolan N, Hausberger M, Wolff A (1997) Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behav Processes 41:257–266. https://doi.org/10.1016/S0376-6357(97)00052-1
Marliani G, Sprocatti I, Schiavoni G, Bellodi A, Accorsi PA (2021) Evaluation of Horses’ daytime activity budget in a model of ethological stable: a case study in Italy. J Appl Anim Welf Sci 24:200–213. https://doi.org/10.1080/10888705.2020.1857252
McDonnell SM (2003) The equid ethogram: A practical field guide to horse behavior. Eclipse Press, Lexington
Mendonça RS, Pinto P, Inoue S, Ringhofer M, Godinho R, Hirata S (2021) Social determinants of affiliation and cohesion in a population of feral horses. Appl Anim Behav Sci 245:105496. https://doi.org/10.1016/j.applanim.2021
Narciso MHPM, da Luz MPF, Maia CM, Nicolau J, Filho PP (2021) Dominance and leadership in the equine social structure: a preliminary study about mules and sex influence. J Equine Vet Sci 99:103392. https://doi.org/10.1016/j.jevs.2021.103392
Reid K, Rogers CW, Gronqvist G, Gee EK, Bolwell CF (2017) Anxiety and pain in horses measured by heart rate variability and behavior. J Vet Behav 22:1–6. https://doi.org/10.1016/j.jveb.2017.09.002
Ricci-Bonot C, Romero T, Nicol C, Mills D (2021) Social buffering in horses is influenced by context but not by the familiarity and habituation of a companion. Sci Rep 11:886. https://doi.org/10.1038/s41598-021-88319-z
Roberts K, Hemmings AJ, Moore-Colyer M, Parker MO, McBride SD (2016) Neural modulators of temperament: a multivariate approach to personality trait identification in the horse. Physiol Behav 167:125–131. https://doi.org/10.1016/j.physbeh.2016.08.029
Rothmann J, Christensen OF, Søndergaard E, Ladewig J (2014) Behavior observation during conformation evaluation at a field test for danish warmblood horses and associations with rideability and performance traits. J Equine Vet Sci 34:288–293. https://doi.org/10.1016/j.jevs.2013.06.007
Seaman SC, Davidson HPB, Waran NK (2002) How reliable is temperament assessment in the domestic horse (Equus caballus)? Appl Anim Behav Sci 78:175–191. https://doi.org/10.1016/S0168-1591(02)00095-3
Søndergaard E, Jensen MB, Nicol HJ (2011) Motivation for social contact in horses measured by operant conditioning. Appl Anim Behav Sci 132:131–137. https://doi.org/10.1016/j.applanim.2011.04.007
Squibb K, Griffin K, Favier R, Ijichi C (2018) Poker Face: discrepancies in behaviour and affective states in horses during stressful handling procedures. Appl Anim Behav Sci 202:34–38. https://doi.org/10.1016/j.applanim.2018.02.003
Stachurska A, Wiśniewska A, Kędzierski W, Różańska-Boczula M, Janczarek I (2021) Behavioural and physiological changes in a herd of arabian mares after the separation of individuals differently ranked within the dominance hierarchy. Animals 11:2694. https://doi.org/10.3390/ani11092694
Vidament M, Lansade L, Danvy S, Priest BDS, Sabbagh M, Ricard A (2021) Personality in young horses and ponies evaluated during breeding shows: phenotypic link with jumping competition results. J Vet Behav 44:1–11. https://doi.org/10.1016/j.jveb.2020.09.003
Villalba JJ, Manteca X, Provenza FD (2009) Relationship between reluctance to eat novel foods and open-field behavior in sheep. Physiol Behav 96:276–281. https://doi.org/10.1016/j.physbeh.2008.10.010
Visser EK, van Reenena CG, van der Werf JTN, Schilderc MBH, Knaapd JH, Barneveld A, Blokhuis HJ (2002) Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol Behav 76:289–296. https://doi.org/10.1016/s0031-9384(02)00698-4
Wolff A, Hausberger M, Le Scolan N (1997) Experimental tests to assess emotionality in horses. Behav Processes 40:209–221. https://doi.org/10.1016/s0376-6357(97)00784-5
Wolter R, Stefanski V, Krueger K (2018) Parameters for the analysis of social bonds in horses. Animals 8:191. https://doi.org/10.3390/ani8110191
Yarnell K, Hall C, Royle C, Walker SL (2015) Domesticated horses differ in their behavioural and physiological responses to isolated and group housing. Physiol Behav 143:51–57. https://doi.org/10.1016/j.physbeh.2015.02.040
Żelazna R, Jezierski T (2018) Behavioural reactions of horses (Equus caballus) to separation stress in conspecifics. A pilot study on emotional contagion in the horse. Anim Sci Pap Rep 36:333–338. https://doi.org/10.3390/fi13100250
Funding
This research was supported by project no. SD/61/ZiR/2022 provided by University of Life Sciences in Lublin, Poland.
Author information
Authors and Affiliations
Contributions
The conception for the paper was conceived by WJ and IW. The experiments were designed by WJ and IW and performed by WJ. The data were analyzed by WJ and TP. The paper was written by WJ and was revised critically by IW and TP. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
All experimental procedures were carried out in accordance with the European directive 2010/63/EU and Polish laws—the Act of January 23, 2021—Law on the organization of breeding and reproduction of farm animals (Journal of Laws 2021, Item 36) and were approved by the Local Ethics Review Committee for Animal Experiments (no 58/2022), Lublin, Poland.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Janicka, W., Wilk, I. & Próchniak, T. Does social motivation mitigate fear caused by a sudden sound in horses?. Anim Cogn 26, 1649–1660 (2023). https://doi.org/10.1007/s10071-023-01805-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-023-01805-x