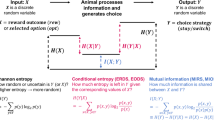
Abstract
This is a review of the reversed-reward contingency (RRC) paradigm in animals and the cognitive functions on which it is founded. I shall present the RRC basic paradigm and the ensuing modifications it underwent, the animals tested, the results obtained and the analyses offered within the literature. Then I would the claim that RRC is a case of a compound cognitive behavior, one that is the result of interactions between three other cognitive functions: crude numerical assessment and economic choice (uniting value assignment and behavioral inhibition). I will present data concerning these three fields and will demonstrate how they are both affecting and affected by the findings of the RRC scheme. RRC is treated here as a test case for a broader type of analysis, one which, hopefully, will show that in order to fully understand composite and complex behavior we need to meticulously explore its building blocks and their dynamic interplay.
Similar content being viewed by others
Notes
Based on one of my commentators, it should be noted that the objective of Boysen and Berntson was not to train their Ss to master the task in any way possible, as has become the case in later studies.
There were no sex and age differences found in the RRC experiments.
The social system difference argument is reiterated by Suda and Call (2004).
It is puzzling that in their concluding paragraph Vlamings et al. claim to have demonstrated that the great apes can master the RRC task without modification.
The lingo in this discourse has many shortcomings and to further complicate matters, in this low extreme of the numerosity assessment scale it is very hard to differentiate number as the salient factor over physical attributes of the stimulus. As noted by one commentator, RNJ is at many times exchangeable with RQJ (relative quantity judgment) but such a term does not exist and RNJ is a cumbersome term. That said the important take home message is that RRC involves a very elementary, low-level numerosity assessment skill, yet still a skill which resides within the realm of numerosity assessment.
It is important to remember that only Boysen et al. subjects were numerosity experienced. However, the interpretation of this fact is not easy, since it is obvious that this did not facilitate spontaneous success.
This is in accord with Beran et al. (2008), see concluding comments.
One referee suggests that the above chance level performance under quality as the salient factor negates the operation of RNJ. Since only one paper addressed this issue I would be hesitant to concur. Drawing upon Padoa-Schioppa et al. (2006) demonstration that economic choice occurs for both quality and quantity I think it is safe to assume that the composite behavioral mechanism that emerged to solve this task can handle both quantity and quality based values.
It is important to note that there is a disagreement as to the specific topographical identification of the OFC since there is a conflict between the definition based upon absolute spatial location and the one incorporating architectonic differentiation within the PFC (Roberts and Wallis 2000).
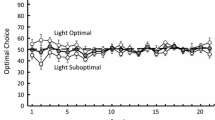
As duly noted by one of my commentators, in RRC as the smaller array diminishes the less appealing it is to the animal from the get go. This may explain why sets where 0 and 1 are in the “correct” array are more difficult.
Boysen et al. (1996) had worked with stimuli pairs containing an empty set versus a content set, but they do not supply us with specific data that would indicate particular performance.
How a system differentiates between agents of plasticity and agents of canalization originating from the same pool of environmental cues is a complex issue far beyond the scope of this review.
References
Agrillo C, Dadda M, Serena G, Bisazza A (2008) Do fish count? Spontaneous discrimination of quantity in female mosquitofish. Anim Cogn 11(3):495–503
Albaich-Serrano A, Guillén-Salazar F, Call J (2007) Mangabeys (Cercocebus torquatus lunulatus) solve the reverse contingency task without a modified procedure. Anim Cogn 10:387–396
Anderson ML (2006) Evidence for massive redeployment of brain areas in cognitive function. Proc Cogn Sci Soc 28:24–29
Anderson ML (2007a) Evolution of cognitive function via redeployment of brain areas. Neuroscientist 13(1):13–21
Anderson ML (2007b) Massive redeployment, exaptation, and the functional integration of cognitive operations. Synthese 159(3):329–345
Anderson JR, Awazu S, Fujita K (2000) Can squirrel monkeys (Saimiri sciureus) learn self-control? A study using food array selection tests and reverse-reward contingency. J Exp Psychol Anim Behav Process 26(1):87–97
Anderson JR, Awazu S, Fujita K (2004) Squirrel monkeys (Saimiri sciureus) choose smaller food arrays: long-term retention, choice with nonpreferred food, and transposition. J Comp Psychol 118(1):58–64
Beran MJ, Evans TA, Harris EH (2008) Perception of food amounts by chimpanzees based on the number, size, contour length and visibility of items. Anim Behav 75(5):1793–1802
Biro D, Matsuzawa T (2001) Use of numerical symbols by the chimpanzee (Pan troglodytes): Cardinals, ordinals, and the introduction of zero. Anim Cogn 4(3–4):193–199
Boysen ST, Berntson GG (1989) Numerical competence in a chimpanzee (Pan troglodytes). J Comp Psychol 103(1):23–31
Boysen ST, Berntson GG (1995) Responses to quantity––perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes). J Exp Psychol Anim Behav Process 21(1):82–86
Boysen ST, Bernston GG, Hannan MB, Cacioppo JT (1996) Quantity-based interference and symbolic representations in chimpanzees (Pan troglodytes). J Exp Psychol Anim Behav Process 22:76–86
Boysen ST, Mukobi KL, Berntson GG (1999) Overcoming response bias using symbolic representations of number by chimpanzees (Pan troglodytes). Anim Learn Behav 27(2):229–235
Boysen ST, Berntson GG, Mukobi KL (2001) Size matters: impact of item size and quantity on array choice by chimpanzees (Pan troglodytes). J Comp Psychol 115(1):106–110
Brannon EM, Roitman J (2003) Nonverbal representations of time and number in non-human animals and human infants. In: Meck W (ed) Functional and neural mechanisms of interval timing. CRC Press, New York, pp 143–182
Chittka L, Geiger K (1995) Can honey-bees count landmarks. Anim Behav 49(1):159–164
Chudasama Y, Kralik J, Murray E (2007) Rhesus monkeys with orbital prefrontal cortex lesions can learn to inhibit prepotent responses in the reversed reward contingency task. Cereb Cortex 17(5):1154–1159
Dacke M, Srinivasan MV (2008). Evidence for counting in insects. Anim Cogn. doi:10.1007/s10071-008-0159-y
Dehaene S (2002) Single-neuron arithmetic. Science 297(5587):1652–1653
Dehaene S, Changeux JP (1993) Development of elementary numerical abilities: a neuronal model. J Cogn Neuro 5(4):390–407
Evans TA, Beran MJ (2007) Delay of gratification and delay maintenance by rhesus macaques (Macaca mulatta). J Gen Psychol 134(2):199–216
Genty E, Roeder JJ (2006) Self-control: why should sea lions, Zalophus californianus, perform better than primates? Anim Behav 72(6):1241–1247
Genty E, Roeder JJ (2007) Transfer of self-control in black (Eulemur macaco) and brown (Eulemur fulvus) lemurs: choice of a less preferred food item under a reverse-reward contingency. J Comp Psychol 121(4):354–362
Genty E, Palmier C, Roeder JJ (2004) Learning to suppress responses to the larger of two rewards in two species of lemurs, Eulemur fulvus and E. macaco. Anim Behav 67:925–932
Godin JG, Keenleyside M (1984) Foraging on patchily distributed prey by a cichlid fish (Teleostei, Cichlidae): a test of the ideal free distribution theory. Anim Behav 32:120–131
Gould SJ, Vrba E (1982) Exaptation: a missing term in the science of form. Paleobiology 8(1):4–15
Hauser MD (1999) Perseveration, inhibition and the prefrontal cortex: a new look. Curr Opin Neurobiol 9(2):214–222
Hayden BY, Platt ML (2007) Animal cognition––great apes wait for grapes. Curr Biol 17(21):R922–R923
Hoare DJ, Couzin ID, Godin JGJ, Krause J (2004) Context-dependent group-size choice in fish. Anim Behav 67(1):155–164
Iversen SD, Mishkin M (1970) Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11(4):376–386
Jones B, Mishkin M (1972) Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol 36(2):362–377
Kalenscher T, Ohmann T, Güntürkün O (2006) The neuroscience of impulsive and self-controlled decisions. Int J Psychophysiol 62:203–211
Karban R, Black CA, Weinbaum SA (2000) How 17-year cicadas keep track of time. Ecol Lett 3(3):253–256
Kralik JD (2005) Inhibitory control and response selection in problem solving: how cotton-top tamarins (Saguinus oedipus) overcome a bias for selecting the larger quantity of food. J Comp Psychol 119(1):78–89
Kralik JD, Hauser MD, Zimlicki R (2002) The relationship between problem solving and inhibitory control: cotton-top tamarin (Saguinus oedipus) performance on a reversed contingency task. J Comp Psychol 116(1):39–50
Krueger LE (1989) Reconciling Fechner and Stevens: toward a unified psychophysical law. Behav Brain Sci 12(2):251–267
Kudo H, Dunbar RIM (2001) Neocortex size and social network size in primates. Anim Behav 62(4):711–722
Leppik EE (1953) The ability of insects to distinguish number. Am Nat 87(835):229–236
Mischel W (1974) Processes in delay of gratification. In: Berkowitz L (ed) Adavnces in experimental social psychology. Academic Press, New York, pp 249–292
Murray EA, Kralik JD, Wise SP (2005) Learning to inhibit prepotent responses: successful performance by rhesus macaques, Macaca mulatta, on the reversed-contingency task. Anim Behav 69:991–998
Nieder A (2005) Counting on neurons: the neurobiology of numerical competence. Nat Rev Neurosci 6(3):1–14
Nieder A, Merten K (2007) A labeled-line code for small and large numerosities in the monkey prefrontal cortex. J Neurosci 27(22):5986–5993
Nieder A, Miller EK (2003) Coding of cognitive magnitude: compressed scaling of numerical information in the primate prefrontal cortex. Neuron 37(1):149–157
Nieder A, Miller EK (2004a) A parieto-frontal network for visual numerical information in the monkey. PNAS 101(19):7457–7462
Nieder A, Miller EK (2004b) Analog numerical representations in rhesus monkeys: evidence for parallel processing. J Cogn Neurosci 16(5):889–901
Nieder A, Freedman DJ, Miller EK (2002) Representation of the quantity of visual items in the primate prefrontal cortex. Science 297(5587):1708–1711
Nieder A, Diester I, Tudusciuc O (2006) Temporal and spatial enumeration processes in the primate parietal cortex. Science 313(5792):1431–1435
Padoa-Schioppa C (2007) Orbitofrontal cortex and the computation of economic value. Ann N Y Acad Sci 1121:232–253
Padoa-Schioppa C, Assad JA (2006) Neurons in the orbitofromtal cortex encode economic value. Nature 441(7090):223–226
Padoa-Schioppa C, Assad JA (2008) The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci 11(1):95–102
Padoa-Schioppa C, Jandolo L, Visalberghi E (2006) Multi-stage mental process for economic choice in capuchins. Cognition 99(1):B1–B13
Pepperberg IM (2006) Grey parrot (Psittacus erithacus) numerical abilities: addition and further experiments on a zero-like concept. J Comp Psychol 120(1):1–11
Pepperberg IM, Gordon JD (2005) Number comprehension by a grey parrot (Psittacus erithacus), including a zero-like concept. J Comp Psychol 119(2):197–209
Reznikova ZI, Ryabko BY (2001) A study of ants’ numerical competence. Elec Trans Art Intel 5(Sect B):111–126
Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS (2004) Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 56(2):129–140
Roberts AC, Wallis JD (2000) Inhibitory control and affective processing in the prefrontal cortex: neuropsychological studies in the common marmoset. Cereb Cortex 10:252–262
Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19(20):9029–9038
Rosati AG, Stevens JR, Hare B, Hauser MD (2007) The evolutionary origins of human patience: temporal preferences in chimpanzees, bonobos, and human adults. Curr Biol 17:1663–1668
Shumaker RW, Palkovich AM, Beck BB, Guagnano GA, Morowitz H (2001) Spontaneous use of magnitude discrimination and ordination by the orangutan (Pongo pygmaeus). J Comp Psychol 115(4):385–391
Silberberg A, Fujita K (1996) Pointing at smaller food amounts in an analogue of Boysen and Berntson’s (1995) procedure. J Exp Anal Behav 66(1):143–147
Stevens JR, Hallinan EV, Hauser MD (2005) The ecology and evolution of patience in two New world monkeys. Biol Lett 1(2):223–226
Suda C, Call J (2004) Piagetian liquid conservation in the Great Apes (Pan paniscus, Pan troglodytes, and Pongo pygmaeus). J Comp Psychol 118(3):265–279
Sulkowski GM, Hauser MD (2001) Can rhesus monkeys spontaneously subtract? Cognition 79(3):239–262
Tegeder RW, Krause J (1995) Density dependence and numerosity in fright stimulated aggregation behaviour of shoaling fish. Philos Tran R Soc Lond B Biol Sci 350(1334):381–390
Tomasello M, Call J (1997) Primate cognition. Oxford University Press, New York
Tudusciuc O, Nieder A (2007) Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. PNAS 104(36):14513–14518
Uher J, Call J (2008) How the great apes (Pan troglodytes, Pongo pygmaeus, Pan paniscus, Gorilla gorilla) perform on the reversed reward contingency task II: transfer to new quantities, long-term retention, and the impact of quantity ratios. J Comp Psychol 122(2):204–212
Uller C, Jaeger R, Guidry G, Martin C (2003) Salamanders (Plethodon cinereus) go for more: rudiments of number in an amphibian. Anim Cogn 6(2):105–112
Vlamings PHJM, Uher J, Call J (2006) How the great apes (Pan troglodytes, Pongo pygmaeus, Pan paniscus, and Gorilla gorilla) perform on the reversed contingency task: the effects of food quantity and food visibility. J Exp Psychol Anim Behav Process 32(1):6–70
Waddington CH (1957) The strategy of the genes. Allen & Unwin, London
Waddington CH (1959) Biological organization: cellular and subcellular. Pergamon Press, New York
Waddington CH (1962) Genetics and development. Columbia University Press, London
Wallis JD, Miller EK (2003) Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci 18(7):2069–2081
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Acknowledgments
I would like to thank prof. Eva Jablonka for reviewing the manuscript and offering insightful corrections and guidance. I would also like to thank deeply two of my referees for their thorough reading and highly constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shifferman, E.M. Its own reward: lessons to be drawn from the reversed-reward contingency paradigm. Anim Cogn 12, 547–558 (2009). https://doi.org/10.1007/s10071-009-0215-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-009-0215-2