Abstract
Chitosan-ZnO nanoparticle (ZnONP) edible coating was applied to extend shelf life of wild-simulated Korean ginseng root (WsKG). In antimicrobial testing of various coating solutions (0.01, 0.02, 0.03% ZnONP), Bacillus cereus (Gram-positive) and Escherichia coli (Gram-negative) were most inhibited by the 0.03% chitosan-ZnONP solution. The 0.03% chitosan-ZnONP solution was finally used for edible coating of WsKG. In SEM analysis, the coat of chitosan and ZnONP was well-formed on the surface of WsKG. In isothermal storage tests (temperature: 5–20 °C, RH: 95%), microbial limit (4.70 log CFU/g) of total aerobic bacteria for non-coated and coated WsKG were reached at 3.9 and 6.3 weeks at 5 °C, 1.9 and 4.3 weeks at 10 °C, and 1.3 and 2.0 weeks at 20 °C, respectively. Mold occurred in the non-coated sample at 4 weeks at 5 °C, but not in the coated sample during 6 weeks. Chitosan-ZnONP edible coating was very effective in preserving WsKG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild-simulated Korean ginseng root (Panax ginseng C.A. Meyer; WsKG) is a botanical plant belonging to the genus Panax within the family Araliaceae (Kim et al., 2019). WsKG is a forest product with various human health benefits, such as lowering blood pressure, anti-cancer, antioxidant, liver detoxification, and lowering lipids (Hong et al., 2008; Kim and Kim, 2006; Kim et al., 2004; Kwon et al., 2003; Yun et al., 2004). The plant presents a higher ginsenoside content compared with ginseng and wild ginseng (Blix et al., 1957), and it was recognized as the second most effective in terms of pharmacological activity after natural wild ginseng (Kim et al., 2007). However, it is one of the forest products that are prone to deterioration due to its high moisture content (Chun and Song, 2007; Kim et al., 2002). Therefore, it is important to minimize the quality loss of WsKG during the distribution process until it is delivered to the consumer.
Ginseng is generally packaged in a cardboard box or polypropylene bag for wholesale, and a polyethylene bag or a box made of manila cardboard at the consumer stage (Park et al., 2000). However, these methods are insufficient to maintain product quality. Deterioration can be suppressed when controlled atmosphere and modified atmosphere packaging methods are used (Hu et al., 2004a; Jeon and Lee, 1999; Kwon et al., 1994). Lee et al. (1976) observed changes in skin and tissue quality of vacuum-packed ginseng roots when fresh ginseng was frozen at −40 °C and stored at −20 °C for 3 months. Jin et al. (2017) extended the shelf life of fresh ginseng roots to 38 weeks by combining sanitizer washing, edible coating (0.5% chitosan and three organic acids), and modified atmosphere packaging.
An antimicrobial coating reduces the exchange of water, O2, and CO2 between food and the environment, and inhibits respiration and oxidation reactions (Min and Krochta, 2005; Robles-Sánchez et al., 2013). This food preservation methods improve the stability, quality, and functionality of food by interfering with the growth of unfavorable microorganisms in the food during storage, transportation, processing (Mellinas et al., 2016). Application of chitosan coatings to fresh food has proven successful in delaying the ripening of strawberries (Hernández-Muñoz et al., 2006), as well as slowing the respiration and browning extent of mushrooms (Liu et al., 2019). Chitosan is well recognized for its intrinsic antimicrobial activity against fungi and some bacteria (Doores, 1993; 2002; Kong et al., 2010; Romanazzi et al., 2013). However, the single chitosan-based coating has the disadvantage of inhibiting only limited microorganisms (Ravi, 2000). Other limitations of chitosan are its low mechanical, barrier and processing properties, and high cost compared with plastic films (Tunc and Duman, 2011).
Various metal nanoparticles (NP), such as silver, gold, titanium oxide, copper oxide, magnesium oxide, and zinc oxide (ZnO), exhibit antibacterial properties (Beyth et al., 2015; Zhang, 2015). ZnONP have been used in the food packaging industry because of their antibacterial properties against a wide range of microorganisms (Espitia et al., 2013; Li et al., 2009). ZnONP can be mixed with a polymer film to prepare antibacterial nanocomposite packaging films that preserve food by preventing the growth of microorganisms on the food surface (Espitia et al., 2013; Soares et al., 2009; Yu et al., 2009). Highly reactive oxygen species, such as peroxide ions, hydrogen peroxide, singlet oxygen, and hydroxyl and superoxide radicals are generated in the presence of ZnONP (Li et al., 2009; Paisoonsin et al., 2013; Tankhiwale and Bajpai, 2012). The production of hydroperoxide can disrupt microbial homeostasis and destroy it. ZnO is partially dissociated by acetic acid to form zinc ions (Zn2+). This partially dissociated Zn2+ also plays a role in antibacterial activity by disturbing the homeostasis of microorganisms and entering the cells, causing cytotoxicity (Pasquet et al., 2014). ZnO nanocomposite packaging has been effectively applied to kiwifruit, apple, mango, and papaya to extend the shelf life (Lavinia et al., 2020; Li et al., 2011; Meindrawan et al., 2018; Meng et al., 2014). Besides, acting as an additive in food, ZnO has been applied to supplement and strengthen nutrients (Shi and Gunasekaran, 2008; Suyatma et al., 2014).
In this study, chitosan-ZnONP edible coating was newly applied to extend shelf life of the WsKG. First, an antimicrobial experiment was conducted to determine the ZnO concentration of the coating solution. The aggregation of ZnONP undesirable for the antimicrobial effect, was examined through particle size analysis. The distribution of chitosan and ZnONP in the WsKG coating was analyzed by SEM. Finally, the antimicrobial effect of the chitosan-ZnONP coating was evaluated based on general bacteria analysis and observation of appearance of WsKG during storage.
Materials and methods
Materials
The WsKG used in this study was 6-year-old root grown in Sansam Village (Wanju, Korea). Chitosan (75–85% deacetylated), acetic acid solution, and ZnONP (average particle size of 20 ± 5 nm) were purchased from Sigma-Aldrich (Seoul, Korea). K2SO4 and CaCl2 were purchased from Daejung chemicals & Metals Co. (Siheung, Korea). Escherichia coli ATCC 25,922 and Bacillus cereus ATCC 11,778 were brought from the American Type Culture Collection (ATCC, Seoul, Korea). E. coli and B. cereus were cultivated at 37 °C for 16 h by picking one inoculation loop and spreading on Luria–Bertani (LB) medium (BD DIFCO, New Jersey, USA). Microbial stock was prepared by inoculating LB broth and 50% glycerol (6:4 v/v) with the bacteria, which was stored at −80 °C.
Chitosan-ZnONP coating of WsKG
The chitosan-ZnONP solution was prepared by referring to Kanmani and Rhim (2014) with modifications. Briefly, 0.03 g ZnONP was dissolved in 0.1 L of 1% acetic acid solution. In another flask, 3 g chitosan was dissolved in 0.3 L of 1% acetic acid solution at 60 °C. The two solutions were mixed and stirred at 80 °C for 5 min, then cooled to room temperature and used for coating. WsKG washed with distilled water was immersed in a 1% CaCl2 solution for 5 min. Drained WsKG was immersed in the chitosan-ZnONP solution for 5 min, drained and left at room temperature for 4 h.
Antimicrobial and physical characterization of chitosan-ZnONP solution and coating
The chitosan-ZnONP solution (0.01, 0.02, 0.03% w/v ZnONP) was added at 0.1% to LB broth and vortexed. A 50-mL aliquot of the LB broth was added to a 100-mL Erlenmeyer flask, followed by 50 μL of the microbial stock, and the mixture cultured at 37 °C for 24 h with agitation at 200 rpm (× 1.4 g) in a thermoshaker incubator (Allsheng Instruments Co., Hangzhou, China). Microbial concentration was monitored by recording the optical density (OD600 nm) at certain intervals using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) (Porel et al., 2011). Results were reported as the average of three repeated measurement.
The particle size distribution and polydispersity index (PDI) of ZnONP were obtained using a zeta-potential analyzer (ELS-8000, OTSUKA, Tokyo, Japan) at 25 °C. Prepared by putting 1 mL of chitosan-ZnONP solution (0.03%) in an Eppendorf tube. Its measurement principle is based on dynamic light scattering (DLS) (Bhattacharjee, 2016). The incident beam was a helium–neon (He–Ne) ion laser at 630 nm. The refractive index and viscosity of the solution were 2.1 and 0.89 cP, respectively.
The coated and non-coated WsKG were observed under a SEM (Hitachi S-3000 N, Hitachi Instruments, Tokyo, Japan) operating at 20 kV and 180 × magnification to observe the microstructure of the chitosan-ZnONP coating. The coated and non-coated WsKG were placed in each Petri dish and evaporated at room temperature.
Storage test of chitosan-ZnONP coated WsKG
Twenty WsKG (coated or non-coated) was stored over a K2SO4 saturated salt solution (95% RH, equivalent to humid storage condition) in a desiccator (internal diameter, 240 mm) at 5, 10, and 20 °C for 6 weeks. One WsKG from each storage condition was removed at certain intervals and placed in a sterile bag with peptone water (0.1% sterile peptone, w/v) of 10 times weight of the ginseng, and homogenized with a stomacher (Bagmixer®400, Interscience, Paris, France) for 90 s. The stepwise-diluted sample was dispensed into 3 M Petrifilm™ (aerobic count plate; 3 M, Seoul, Korea) and cultured at 37 °C for 48 h, followed by the enumeration of colonies (n = 3). The microbial concentration was expressed in log CFU/g units calculated by considering the dilution factor, the amount of peptone, and the weight of WsKG.
The appearance of WsKG was qualitatively observed with the naked eye, given in photographs.
Results and discussion
ZnONP size in coating solution
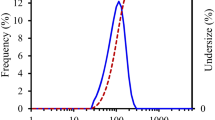
When ZnONP was dispersed at 0.01, 0.02, and 0.03% (w/v) in the acetic acid solution used for dissolving chitosan, the average particle size was 106.5, 94.2, and 92.7 nm, showing similar sized NPs (Fig. 1). It has been reported that the average particle size decreases as the concentration of ZnONP increases (Kołodziejczak-Radzimska et al., 2012). The original size before dispersion was 20 ± 5 nm but increased after dispersion in the solution because of the ZnONP aggregation (Shi and Gunasekaren, 2008). The ZnONP aggregation were also observed in the preparation of the films of ZnONP incorporated chitosan (Lavinia et al., 2020) and pectin (Suyatma et al., 2014). The ZnONP of 0.03% was considered to have the best antimicrobial effect because it was the smallest in particle size and therefore the largest in surface area favorable for its reactivity. According to Webster and Seil (2012), the specific surface area of nanoparticles increases with decreasing particle size, allowing greater material interaction with the surrounding environment. Therefore, for intrinsic antibacterial materials such as zinc and silver, increasing the surface-to-volume ratio enhances the antibacterial effect.
The PDI of ZnONPs of 0.01, 0.02, and 0.03% were 0.385, 0.251, and 0.116, respectively. It has been reported that the PDI decreases as the concentration of ZnONP increases (Lavinia et al., 2020). PDI means the degree of non-uniformity of a particle size distribution. PDI of < 0.1, 0.1–0.4, and > 0.4 indicate highly monodisperse, moderately polydisperse, and highly polydisperse, respectively (Bhattacharjee, 2016). Therefore, the ZnONPs were considered to have a moderate size distribution.
Antimicrobial property of chitosan-ZnONP coating solution
The microorganisms that can deteriorate ginseng are known as B. cereus, E. coli, Staphylococcus aureus, etc. (Kim and Yang, 2018). Among them, B. cereus and E. coli were applied to this study. As the amount of ZnO in the coating solution increased from 0.00 to 0.01, 0.02, and 0.03% (w/v), the maximum OD600 nm (equivalent to microbial content) of B. cereus growth decreased from 2.60 to 2.04, 1.96, and 1.87, respectively (Fig. 2). In the case of E. coli, the maximum OD600 nm were 2.16, 1.90, 1.65, and 1.42, respectively. As a result, it was found out that as the amount of ZnO increased, the growth of microorganisms was better inhibited.
Bacillus cereus and E. coli reached the maximum OD600 nm after 9 and 7 h, respectively. B. cereus and E. coli are Gram-positive bacteria and Gram-negative bacteria. In Gram-positive bacteria, the cell wall is composed of multilayers of peptidoglycan with an abundance of pores through which the ZnO particles could enter the cells. By contrast, Gram-negative bacteria have a thin peptidoglycan layer, but a complex cell wall structure, which would be less susceptible to attack by NP (Anitha et al., 2012). Therefore, the NP have more efficacious antimicrobial activity against Gram-positive bacteria with simple cell walls than Gram-negative bacteria with complex cell walls (Anitha et al., 2012; Paisoonsin et al., 2013). In contrast, Rhim and Wang (2013) and Li et al. (2009) reported that the thick layer of peptidoglycan inhibited ZnONP from penetrating cells of Gram-positive bacteria, while ZnONP could easily penetrate the thin peptidoglycan layer of Gram-negative bacteria. In this study, the findings were consistent with the ZnONP being more effective against B. cereus.
Microstructure of chitosan-ZnONP coating film
SEM is used to study the surface morphology of the NP composite films (Akhtar et al., 2018). In the non-coated WsKG, a wrinkled surface corresponding to WsKG peel was observed, but the coated WsKG had agglomerated particles on its coat (Fig. 3). The coating thickness or content could not be presented, because, unlike foods such as apples, WsKG did not have a constant surface curvature and roughness, and its surface area was not constant even with the same weight, resulting in too much variation. In general, when metal NPs are identified through SEM, they are observed to be irregular and lumpy (Plascencia-Villa et al., 2012; Tailor et al., 2019). In contrast, chitosan appears to have a uniform shape without lumps (Paul et al., 2018). For instance, TiO2NP appears as agglomerated particles distributed on the chitosan matrix in the microstructure of chitosan-TiO2NP composite film (Xing et al., 2020). Accordingly, it was judged that the agglomerated particles on the WsKG coat are ZnONP dispersed in chitosan. As a result, it was found that the chitosan-ZnONP was well-formed in the coated WsKG. The chitosan-ZnONP coating on fresh-cut papaya has been reported to show antimicrobial activity (Lavinia et al., 2020).
Shelf life of chitosan-ZnONP coated WsKG
The antimicrobial effect on WsKG coated with chitosan-ZnONP (0.03%) solution, which was found to have the greatest antibacterial effect, was analyzed. The growth of microorganisms (total aerobic bacteria) was inhibited in coated WsKG more than non-coated WsKG (Fig. 4). Coated WsKG decreased in the microbial concentration until about 4 h of storage (5, 10, and 20 °C). It shows that the antimicrobial effect was markedly higher than the growth effect in the early stages of growth. Afterwards, the microbial concentration began to increase, which seems to be a phenomenon that appeared as the growth effect increased. At a low temperature of 5 °C, the growth effect was lower than at a high temperature of 20 °C, so it seems that the microbial concentration increased relatively slowly. Overall, the microbial limit of WsKG (4.70 log CFU/g; Ministry of Agriculture, Food and Rural Affairs, 2017) was reached faster as the storage temperature increased. That is, non-coated and coated WsKG showed 3.9 and 6.3 weeks at 5 °C, 1.9 and 4.3 weeks at 10 °C, and 1.3 and 2.0 weeks at 20 °C, respectively. These values were obtained through linear interpolation between data points. Highly reactive oxygen species, such as peroxide ions, hydrogen peroxide, singlet oxygen, and hydroxyl and superoxide radicals, are generated in the presence of ZnONP (Li et al., 2009; Paisoonsin et al., 2013; Tankhiwale and Bajpai, 2012). ZnONP penetrated the cells of the bacteria, thereby damaging protein, DNA, and lipids, destroying the cell membrane or causing cell lysis, resulting in stress-induced production of free radicals (Paisoonsin et al., 2013; Tankhiwale and Bajpai, 2012; Yousef and Danial, 2012). The preservative effect of ZnONP on fruits has been demonstrated such as apple, kiwifruit, mango and papaya (Lavinia et al., 2020; Li et al., 2011; Meng et al., 2014; Meindrawan et al., 2018).
The changes in the physical appearance of WsKG at 5 °C, a general refrigeration temperature, are shown in Fig. 5. Mold (white spots at main root) was observed at 4 and 6 weeks for the non-coated WsKG, whereas the coated WsKG did not develop mold for 6 weeks. At 6 weeks, the microbial concentration of aerobic bacteria for the coated WsKG was also below the microbial limit (Fig. 4), indicating that the antimicrobial effect was pronounced. Browning at fine root was developed for the non-coated WsKG more than the coated WsKG. Ginseng turns dark brown by natural fermentation when stored for several days (Bae et al., 2014), and it is more prone to browning at higher temperatures (Hu et al., 2004a, b, 2005). This indicates that the natural fermentation of WsKG was delayed by coating, resulting in the browning inhibition. Overall, the coating could suppress WsKG deterioration.
WsKG is a forest product that is well recognized because of its pharmacological activity. However, WsKG is a perishable crop due to exposure to humid condition from distribution to storage. Therefore, it is important to develop a packaging technique that maintains the freshness of WsKG. In this study, the chitosan-ZnONP coating technique, which has not yet been tried, was applied to WsKG. Result of storage experiments revealed that the quality of coated WsKG was better than the non-coated control in both general bacteria and physical appearance analyses, and the shelf life could be extended to 6 weeks at 5 °C. There are several techniques for extending the shelf life of WsKG, such as modified atmosphere packaging, sanitizer washing, and edible coating with chitosan and organic acids. Through this study, it was found that the chitosan-ZnONP coating technique can also be applied to WsKG. In addition, this chitosan-ZnONP coating technique is expected to work effectively on other perishable produces. However, this study only suggested the applicability of this coating technique to WsKG. In the future, it will be necessary to investigate the effects of the coating variables (coating amount, coating method, etc.) in more details.
References
Akhtar K, Khan SA, Asiri AM. Scanning electron microscopy: Principle and applications in nanomaterials characterization. pp. 113-145. In: Handbook of Material Characterization. Sharma S (ed). Springer, Gewerbestr, Switcherland (2018)
Anitha S, Barbu B, John Thiruvadigal D, Gopalakrishnan C, Natarajan TS. Optical, bactericidal and water repellent properties of electrospun nanocomposite membranes of cellulose acetate and ZnO. Carbohydrate Polymer. 87: 1065-1072 (2012)
Bae HJ, Chung SI, Lee SC, Kang MY. Influence of aging process on the bioactive components and antioxidant activity of ginseng (Panax ginseng L.). Journal of Food Science. 79: H2127-H2131 (2014)
Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evidence-Based Complementary and Alternative Medicine. 2015: 246012 (2015)
Bhattacharjee S. DLS and zeta potential – What they are and what they are not?. Journal of Controlled Release. 235: 337-351 (2016)
Blix G, Gottschalk A, Klenk E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature. 179: 1088 (1957)
Chun HH, Song KB. Aqueous chlorine dioxide treatment improves the shelf life of Panax ginseng C.A. Meyer. Preventive Nutrition and Food Science. 12: 284-288 (2007)
Doores S. Oraganic acids. pp. 95-136. In: Antimicrobials in foods. Branen AL, Davidson PM (eds). Marcel Dekker, Inc. New York, USA (1993)
Doores S. pH control agents and acidulants. pp. 621-660. In: Handbook of Food Additives. Branen AL, Davidson PM, Salminen S, Thorngate JH (eds). Marcel Dekker, Inc. New York, USA (2002)
Espitia PJP, Soares NFF, Teofilo RF, Coimbra JSR, Vitor DM, Batista RA, Ferreira SO, Andrade NJ, Medeiros EAA. Physical-mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydrate Polymer. 94: 199-208 (2013)
Hernández-Muñoz P, Almenar E, Ocio MJ, Gavara R. Effect of calcium dips and chitosan coating on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biology and Technology. 39: 247-253 (2006)
Hong MH, Lim HK, Park J, Jun NJ, Cho M, Cho SK. The antihypertensive and vasodilating effects of adventitious root extracts of wild ginseng. Applied Biological Chemistry. 51: 102-107 (2008)
Hu W, Tanaka S, Uchino T, Hamanaka D, Hori Y. Effects of packaging film and storage temperature on the quality of fresh ginseng packaged in modified atmosphere. Journal of the Faculty of Agriculture - Kyushu University. 49: 139-147 (2004a)
Hu W, Tanaka S, Uchino T, Nei D. Storage life extension of ginseng using active modified atmosphere packaging by nitrogen generator. Journal of the Faculty of Agriculture- Kyushu University. 49: 401-408 (2004b)
Hu W, Xu P, Uchino T. Extending storage life of fresh ginseng by modified atmosphere packaging. Journal of the Science of Food and Agriculture. 85: 2475-2481 (2005)
Jeon BS, Lee CY. Shelf-life extension of American fresh ginseng by controlled atmosphere storage and modified atmosphere packaging. Journal of Food Science. 64, 328-331 (1999)
Jin TZ, Huang M, Niemira BA, Cheng L. Microbial reduction and sensory quality preservation of fresh ginseng roots using nonthermal processing and antimicrobial packaging. Journal of Food Processing and Preservation. 41: e12871 (2017)
Kanmani P, Rhim JW. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydrate Polymers. 106: 190-199 (2014)
Kim JH, Kim JK. Antioxidant activity and functional component analysis of Korean mountain ginseng’s different sections. Journal of the Korean Society of Food Science and Nutrition. 35: 1315-1321 (2006)
Kim YR, Yang CS. Protective roles of ginseng against bacterial infection. Microbial Cell. 5: 472-481 (2018)
Kim JH, Koo NS, Kim EH, Sohn HJ. Changes in sensory characteristics and chemical constituents of raw ginseng roots individually packaged in a soft film during storage. Journal of Ginseng Research. 26: 145-150 (2002)
Kim SJ, Shin SS, Seo BI, Ji SY. Effects of mountain grown ginseng radix, mountain cultivated ginseng radix, and cultivated ginseng radix on apoptosis of HL-60 cells. The Korean Journal of Herbology. 19: 41-50 (2004)
Kim YJ, Park HS, Kwon KR, Kim HY. Protective effects of cultivated ginseng, cultivated wild ginseng of Korean and Chinese against CCl4 and t-BHP acute hepatotoxicity in ICR Mice. Journal of Pharmacopuncture. 10: 101-107 (2007)
Kim KY, Jeong DH, Kim HJ, Jeon KS, Kim MJ, Um YR. A study on growth characteristics of wild-simulated ginseng (Panax ginseng C. A. Meyer) by direct seeding and transplanting. Korean Journal of Plant Resources. 32: 160-169 (2019)
Kołodziejczak-Radzimska A, Markiewicz E, Jesionowski T. Structural characterization of ZnO particles obtained by the emulsion precipitation method. Journal of Nanomaterials. 2012: 656353 (2012)
Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology. 144: 51-63 (2010)
Kwon HR, Lee S, Lee DS. Effect of packaging on keeping quality of fresh ginseng. The Korean Society of Food Preservation. 1: 93-98 (1994)
Kwon KR, Cho AL, Lee SG. The study on acute and subacute toxicity and anti-cancer effects of cultivated wild ginseng herbal acupuncture. Journal of Pharmacopuncture. 6: 7-27 (2003)
Lavinia M, Hibaturrahman SN, Harinata H, Wardana AA. Antimicrobial activity and application of nanocomposite coating from chitosan and ZnO nanoparticle to inhibit microbial growth on fresh-cut papaya. Food Research. 4: 307-311 (2020)
Lee YH, Kim KW, Shin HK, Baek CK, Lee C. Studies on the long-term storage of fresh ginseng. Korean Institute of Science & Technology Report I. 23-25 (1976)
Li JH, Hong RY, Li MY, Li HZ, Zheng Y, Ding J. Effects of ZnO nanoparticles on the mechanical and antibacterial properties of polyurethane coatings. Progress in Organic Coating. 64: 504-509 (2009)
Li X, Li W, Jiang Y, Ding Y, Yun J, Tang Y, Zhang P. Effects of nano-ZnO coated active packaging on quality of fresh-cut “Fuji” apple. International Journal of Food Science and Technology. 46: 1947-1955 (2011)
Liu J, Liu S, Zhang X, Kan J, Jin C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biology and Technology. 147: 39-47 (2019)
Meindrawan B, Suyatma NE, Wardana AA, Pamela VY. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packaging and Shelf Life. 18: 140-146 (2018)
Mellinas C, Valdes A, Ramos M, Burgos N, Garrigos MC, Jimenez A. Active edible films: Current state and future trends. Journal of Applied Polymer. 133: 42631 (2016)
Meng X, Zhang M, Adhikari B. The effects of ultrasound treatment and nano-Zinc oxide coating on the physiological activities of fresh-cut kiwifruit. Food and Bioprocess Technology. 7: 126-132 (2014)
Min S, Krochta JM. Antimicrobial films and coatings for fresh fruit and vegetables. pp. 454–485. In: Improving the Safety of Fresh Fruit and Vegetables. Jongen W (ed). CRC Press, New York, USA (2005)
Ministry of Agriculture, Food and Rural Affairs. Enforcement decree of the ginseng industry act, 2017. Available from: https://www.law.go.kr/lsInfoP.do?lsiSeq=194803#J2:0. Accessed Aug. 18, 2021.
Paisoonsin S, Pornsunthorntawee O, Rujiravani R. Preparation and characterization of ZnO-deposited DBD plasma-treated PP packaging film with antibacterial activities. Applied Surface Science. 273: 824-835 (2013)
Park HW, Park JD, Lee HJ, Kim DM. A study on distribution and packaging of Korean fresh ginsengs in domestic markets. Journal of Korean Society of Food Science Nutrition. 29: 1151-1154 (2000)
Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger MA. The contribution of zinc ions to the antimicrobial activity of zine oxide. Colloids and Surfaces A: Physiochemical and Engineering Aspects. 457: 263-274 (2014)
Paul SK, Sarkar S, Sethi LN, Ghosh SK. Development of chitosan based optimized edible coating for tomato (Solanum lycopersicum) and its characterization. Journal of Food Science and Technology. 55: 2446-2456 (2018)
Plascencia-Villa G, Starr CR, Armstrong LS, Ponce A, José-Yacamán M. Imaging interactions of metal oxide nanoparticles with macrophage cells by ultra-high resolution scanning electron microscopy techniques. Integrative Biology. 4: 1358-1366 (2012)
Porel S, Ramakrishna D, Hariprasad E, Dutta Gupta E, Radhakrishnan TP. Polymer thin film with in situ synthesized silver nanoparticles as a potent reusable bactericide. Current Science. 101: 927-934 (2011)
Ravi KMNV. A review of chitin and chitosan applications. Reactive and Functional Polymers. 46: 1-27 (2000)
Rhim JW, Wang LF. Mechanical and water barrier properties of agar/κ-carrageenan/konjac glucomannan ternary blend biohydrogel films. Carbohydrate Polymers. 96: 71-81 (2013)
Robles-Sánchez RM, Rojas-Graü MA, Odriozola-Serrano I, González-Aguilar G, Martin-Belloso O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT – Food Science and Technology. 50: 240-246 (2013)
Romanazzi G, Feliziani E, Santini M, Landi L. Effectiveness of post-harvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biology and Technology. 75: 24-27 (2013)
Shi L, Gunasekaran S. Preparation of pectin-ZnO nanocomposite. Nanoscale Research Letters. 3: 491-495 (2008)
Soares NFF, Pires ACS, Camilloto GP, Santiago-Silva P, Espitia PJP, Silva WA. Recent patents on active packaging for food application. Recent Patents on Food, Nutrition, & Agriculture. 1: 171-178 (2009)
Suyatma NE, Ishitakawa YM, Kitazawa H. Nanoreinforcement of pectin film to enhance its functional packaging properties by incorporating ZnO nanoparticles. Advanced Materials Research. 845: 451-456 (2014)
Tailor G, Chaudhay J, Verma D, Sarma BK. Microscopic study of zinc nanoparticles synthesized using thermosetting polymer. Applied Microscopy. 49: 1-5 (2019)
Tankhiwale R, Bajpai SK. Preparation, characterization and antibacterial application of ZnO-nanoparticles coated polyethylene films for good packaging. Colloids and Surfaces B: Biointerfaces. 90: 16-20 (2012)
Tunc S, Duman O. Preparation of active antimicrobial methyl cellulose/carvacrol/montmorillonite nanocomposite films and investigation of carvacrol release. LWT-Food Science and Technology. 44: 465-472 (2011)
Webster TJ, Seil I. Antimicrobial application of nanotechnology: methods and literature. International Journal of Nanomedicine. 7: 2767-2781 (2012)
Xing Y, Li X, Guo X, Li W, Chen J, Liu Q, Xu Q, Wang Q, Yang H, Shui Y, Bi X. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials. 10: 1365 (2020)
Yousef JM, Danial EN. In vitro antibacterial activity and minimum inhibitory concentration of zinc oxide and nano-particle zinc oxide against pathogenic strains. Journal of Health Sciences. 2: 38-42 (2012)
Yu J, Yang J, Liu B, Ma X. Preparation and characterization of glycerol plasticized-pea starch/ZnO-carboxymethyl cellulose sodium nanocomposites. Bioresource Technology. 100: 2832-2841 (2009)
Yun SN, Moon SJ, Ko SK, Im BO, Chung SH. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Archives of Pharmacal Research. 27: 790-796 (2004)
Zhang X. Gold nanoparticle: Recent advances in the biomedical applications. Cell Biochemistry and Biophysics. 72: 771-775 (2015)
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (121017-03); R&D Convergence Center Support Program of the Ministry of Food, Agriculture, Forestry and Fisheries, Republic of Korea (710013-03); and the Dongguk University Research Fund of 2020-2021.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, S.H., Cha, H.J., Jung, S.W. et al. Application of chitosan-ZnO nanoparticle edible coating to wild-simulated Korean ginseng root. Food Sci Biotechnol 31, 579–586 (2022). https://doi.org/10.1007/s10068-022-01054-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01054-7