Abstract
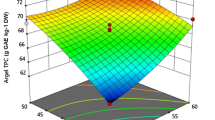
The effects of ultrasound-assisted extraction (UAE) variables—namely extraction temperature (40–60 °C), ultrasonic power (50–150 W), and sonication time (40–60 min)—on the extractive value (EV) of bioactive phenolics from Malva sylvestris leaves were investigated and optimized using Response surface methodology. The effects of extraction solvents (ethanol, ethyl acetate, and n-hexane) on EV, free radical scavenging activity (FRSA), total phenolic content (TPC), and major bioactive phenolics were studied using agitated bed extraction (ABE), and the results were compared with the UAE findings. Under the optimal UAE conditions (48 °C, 110.00 W, and 48.77 min) the experimental EV was 279.89 ± 0.21 mg/g with 71.12 ± 0.15% DPPHsc, 73.35 ± 0.11% ABTSsc, and a TPC of 152.25 ± 0.14 mg GAE/g. Ethanolic ABE results in higher EV (320.16 ± 0.25 mg g−1) compared to UAE, while the FRSA and TPC values were reduced. HPLC analysis revealed that the concentration of bioactive phenolics increased significantly (p < 0.05) under the optimal UAE conditions.


Similar content being viewed by others
References
Oroian M, Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 74: 10–36 (2015)
Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 6: 806–817 (2013)
Martinez JL. Supercritical fluid extraction of nutraceuticals and bioactive compounds, New York. United States of America, CRC Press. (2008)
Feng H, Yang W. Power ultrasound. In: Handbook of food science, technology, and engineering, edited by Hui, Y.H. New York, CRC Press. (2005)
Sharma A, Gupta MN. Oil extraction from almond, apricot and rice by three-phase partitioning after ultrasonication. Eur. J. Lipid Sci. Tech. 106: 183–186 (2004)
Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 17: 300–312 (2006)
Zhou B, Feng H, Lou Y. Ultrasound enhanced sanitizer efficacy in reduction of Escherichia coli O157:H7 population on spinach leaves. J. Food Sci. 74: 308–313 (2009)
Baumann A, Martin SE, Feng H. Removal of Listeria monocytogenes biofilms from stainless steel using ultrasound and ozone. J. Food Prot. 72: 1306–1309 (2009)
Raviyan P, Zhang Z, Feng H. Ultrasonication for tomato pectin methyl esterase inactivation: effect of cavitation intensity and temperature on inactivation. J. Food Eng. 70: 189–196 (2005)
Zhang Z, Feng H, Niu Y, Eckhoff SR. Starch recovery from degermed corn flour and hominy feed using power ultrasound. Cereal Chem. 82: 447–449 (2005)
Zhang HQ, Barbosa-Canovas GV, Balasubramaniam VM, Dunne CP, Farkas DF, Yuan JT. Nonthermal processing technologies for food, United Kingdom, Wiley, IFT Press. (2011)
Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A. Optimization of conventional and ultrasound-assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT Food Sci. Technol. 64: 1114–1122 (2015)
Jian-Bing J, Xiang-Hong L, Mei-Qiang C, Zhi-Chao X. Improvement of leaching process of Geniposide with ultrasound. Ultrason. Sonochem. 13: 455–462 (2006)
Rostagno MA, Palma M, Barroso CG. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 1012: 119–128 (2003)
Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 8: 303–313 (2001)
Cristina Soria A, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 21: 323–331 (2010)
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 34: 540–560 (2017)
Samavatia V, Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its antioxidant activity. Int. J. Biol. Macromolec. 60: 427–436 (2013)
Pirbalouti AG, Yousefi M, Heshmetollah N, Karimi I, Koohpayeh A. Bioactivity of Malva Sylvestris L., a Medicinal Plant from Iran. E. J. Bio. 5: 62–66 (2009)
Wang ZY. Impact of anthocyanin from Malva sylvestris on plasma lipids and free radical. J For Res. 16: 228–232 (2005).
Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Characterization of valuable compounds from winter melon (Benincasa hispida (Thunb.) Cogn.) seeds using supercritical carbon dioxide extraction combined with pressure swing technique. Food Bioprocess Tech. 9: 396–406 (2016)
Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299: 152–178 (1999)
Sehati N, Dalali N, Soltanpour S, Seyed Dorraji MS. Application of hollow fiber membrane mediated with titanium nanowire/reduced grapheme oxide nanocomposite in preconcentration of clotrimazole and tylosin. J. Chromatogr. A 1420: 46–53 (2015)
Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 93: 47–56 (2005)
Weng WL, Liu YC, Lin CW. Studies on the optimum models of the dairy product Kou Woan Lao using response surface methodology. Asian-Australasian J. Anim. Sci. 14: 1470–1476 (2001)
Deng Y, Zhao Y. Effect of pulsed vacuum and ultrasound osmo-pretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT - Food Sci. Technol. 41: 1575–1585 (2008)
He L, Xu H, Liu X, He W, Yuan F, Hou Z. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC-ABTS+ assay. Food Res. Int. 44: 1161–1167 (2011)
Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 56: 9225–9229 (2008)
Gan CY, Latiff AA. Optimization of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 124: 1277–1283 (2011)
Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules 17: 11748–11762 (2012)
Caresa MG, Vargas Y, Gaete L, Sainz J, Alarcon J. Ultrasonically assisted extraction of bioactive principles from Quillaja Saponaria Molina. Phys. Procedia. 3: 169–178 (2010)
Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Ultrasound-assisted extraction of valuable compounds from winter melon (Benincasa hispida) seeds. Int. Food Res. J. 20: 331–338 (2013)
Zhang ZS, Wang LJ, Li D, Jiao SS, Chen Z XD, Mao H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 62: 192–198 (2008)
Sivakumar V, Ravi Verma V, Rao PG, Swaminathan G. Studies on the use of power ultrasound in solid-liquid myrobalan extraction process. J. Clean. Prod. 15: 1813–1818 (2007)
Shekhar UK, Tiwari BK, Smyth TJ, Donnell CP. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed using response surface methodology. Ultrason. Sonochem. 23: 308–316 (2015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bimakr, M., Ganjloo, A., Zarringhalami, S. et al. Ultrasound-assisted extraction of bioactive compounds from Malva sylvestris leaves and its comparison with agitated bed extraction technique. Food Sci Biotechnol 26, 1481–1490 (2017). https://doi.org/10.1007/s10068-017-0229-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0229-5