Abstract
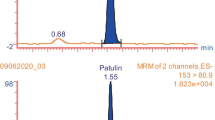
A new effective method was developed to determine the concentration of arbutin in pear peels using a modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method and liquid chromatography-tandem mass spectrometry (LC-MS/MS). The original QuEChERS was modified to enable the extraction of the polar arbutin molecule. Use of an initial 50:50 acetonitrile:water extraction solvent led to the highest extraction efficiency. The arbutin extracted from pear peels was found to be identical to the β-arbutin standard, as confirmed by NMR and LC-MS/MS analyses. For quantitative analysis, the mass spectra of the precursor ion [M+NH4]+ at m/z 290.0 and the product ion of arbutin at m/z 163.0 were used. The limit of detection, limit of quantification, linearity, precision, accuracy, and recovery of the proposed method were evaluated. We successfully applied this method to pear samples and it may be suitable for the quantitative analysis of arbutin in other similar plant materials.
Similar content being viewed by others
References
Rychlinska I, Nowak S. Quantitative determination of arbutin and hydroquinone in different plant materials by HPLC. Not. Bot. Horti Agrobo. 40: 109–113 (2012)
Fiorentino A, Castaldi S, D’Abrosca B, Natale A, Carfora A, Messere A, Monaco P. Polyphenols from the hydroalcoholic extract of Arbutus unedo living in a monospecific Mediterranean woodland. Biochem. Syst. Ecol. 35: 809–811 (2007)
Lukas B, Schmiderer C, Mitteregger U, Novak J. Arbutin in marjoram and oregano. Food Chem. 121: 185–190 (2010)
Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of alpha-arbutin and beta-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci. Biotech. Bioch. 59: 143–144 (1995)
Gallo FR, Pagliuca G, Multari G, Panzini G, D’Amore E, Altieri I. New highperformance liquid chromatography-DAD method for analytical determination of arbutin and hydroquinone in rat plasma. Indian J. Pharm. Sci. 77: 530–535 (2015)
Kurosu J, Sato T, Yoshida K, Tsugane T, Shimura S, Kirimura K, Kino K, Usami S. Enzymatic synthesis of alpha-arbutin by alpha-anomer-selective glucosylation of hydroquinone using lyophilized cells of Xanthomonas campestris WU-9701. J. Biosci. Bioeng. 93: 328–330 (2002)
Robertson JA, Howard LA. Effect of carbohydrates on growth of Ureaplasma urealyticum and Mycoplasma hominis. J. Clin. Microbiol. 25: 160–161 (1987)
Sugimoto K, Nishimura T, Nomura K, Sugimoto K, Kuriki T. Syntheses of arbutin-alpha-glycosides and a comparison of their inhibitory effects with those of alpha-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 51: 798–801 (2003)
Cho JY, Park KY, Lee KH, Lee HJ, Lee SH, Cho JA, Kim WS, Shin SC, Park KH, Moon JH. Recovery of arbutin in high purity from fruit peels of pear (Pyrus pyrifolia Nakai). Food Sci. Biotechnol. 20: 801–807 (2011)
Seo DH, Jung JH, Ha SJ, Song MC, Cha J, Yoo SH, Kim TJ, Baek NI, Park CS. Highly selective biotransformation of arbutin to arbutin-alpha-glucoside using amylosucrase from Deinococcus geothermalis DSM 11300. J. Mol. Catal. BEnzym. 60: 113–118 (2009)
Hu ZM, Zhou Q, Lei TC, Ding SF, Xu SZ. Effects of hydroquinone and its glucoside derivatives on melanogenesis and antioxidation: Biosafety as skin whitening agents. J. Dermatol. Sci. 55: 179–184 (2009)
Couteau C, Coiffard LJ. Photostability determination of arbutin, a vegetable whitening agent. Farmaco 55: 410–413 (2000)
Kwiecien I, Szopa A, Madej K, Ekiert H. Arbutin production via biotransformation of hydroquinone in in vitro cultures of Aronia melanocarpa (Michx.) Elliott. Acta Biochim. Pol. 60: 865–870 (2013)
Schindler G, Patzak U, Brinkhaus B, von Niecieck A, Wittig J, Krahmer N, Glockl I, Veit M. Urinary excretion and metabolism of arbutin after oral administration of Arctostaphylos uvae ursi extract as film-coated tablets and aqueous solution in healthy humans. J. Clin. Pharmacol. 42: 920–927 (2002)
Moran A, Gutierrez S, Martinez-Blanco H, Ferrero MA, Monteagudo-Mera A, Rodriguez-Aparicio LB. Non-toxic plant metabolites regulate Staphylococcus viability and biofilm formation: A natural therapeutic strategy useful in the treatment and prevention of skin infections. Biofouling 30: 1175–1182 (2014)
Zbigniew S, Beata Z, Kamil J, Roman F, Barbara K, Andrzej D. Antimicrobial and antiradical activity of extracts obtained from leaves of three species of the genus pyrus. Microb. Drug Resist. 20: 337–343 (2014)
de Arriba SG, Naser B, Nolte KU. Risk assessment of free hydroquinone derived from Arctostaphylos Uva-ursi folium herbal preparations. Int. J. Toxicol. 32: 442–453 (2013)
Assaf MH, Ali AA, Makboul MA, Beck JP, Anton R. Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Med. 53: 343–345 (1987)
Zhang L, Zhang W, Chen G. Determination of arbutin and bergenin in Bergeniae Rhizoma by capillary electrophoresis with a carbon nanotubeepoxy composite electrode. J. Pharmaceut. Biomed. 115: 323–329 (2015)
Lamien-Meda A, Lukas B, Schmiderer C, Franz C, Novak J. Validation of a quantitative assay of arbutin using gas chromatography in Origanum majorana and Arctostaphylos uva-ursi extracts. Phytochem. Analysis 20: 416–420 (2009)
Jurica K, Karaconji IB, Segan S, Opsenica DM, Kremer D. Quantitative analysis of arbutin and hydroquinone in strawberry tree (Arbutus unedo L., Ericaceae) leaves by gas chromatography-mass spectrometry. Arh. Hig. Rada Toksiko. 66: 197–202 (2015)
Parejo I, Viladomat F, Bastida J, Codina C. A single extraction step in the quantitative analysis of arbutin in bearberry (Arctostaphylos uva-ursi) leaves by high-performance liquid chromatography. Phytochem. Analysis 12: 336–339 (2001)
Pavlovic RD, Lakusic B, Doslov-Kokorus Z, Kovacevic N. Arbutin content and antioxidant activity of some Ericaceae species. Pharmazie 64: 656–659 (2009)
Lin LZ, Harnly JN. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agr. Food Chem. 56: 9094–9101 (2008)
Ferreira D, Guyot S, Marnet N, Delgadillo I, Renard CMGC, Coimbra MA. Composition of phenolic compounds in a Portuguese pear (Pyrus communis L. var. S. Bartolomeu) and changes after sun-drying. J. Agr. Food Chem. 50: 4537–4544 (2002)
Wiilkowska A, Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 125: 803–812 (2011)
Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W, Mastovska K, Hoh E, Leepipatpiboon N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 1217: 2548–2560 (2010)
Lesueur C, Knittl P, Gartner M, Mentler A, Fuerhacker M. Analysis of 140 pesticides from conventional farming foodstuff samples after extraction with the modified QuECheRS method. Food Control 19: 906–914 (2008)
Paya P, Anastassiades M, Mack D, Sigalova I, Tasdelen B, Oliva J, Barba A. Analysis of pesticide residues using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) pesticide multiresidue method in combination with gas and liquid chromatography and tandem mass spectrometric detection. Anal. Bioanal. Chem. 389: 1697–1714 (2007)
Cui T, Nakamura K, Ma L, Li JZ, Kayahara H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in Oriental pear. J. Agr. Food Chem. 53: 3882–3887 (2005)
Taylor VF, March RE, Longerich HP, Stadey CJ. A mass spectrometric study of glucose, sucrose, and fructose using an inductively coupled plasma and electrospray ionization. Int. J. Mass Spectrom. 243: 71–84 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Kim, J.I. & Lee, C.W. Development and validation of a modified QuEChERS method coupled with LC-MS/MS to determine arbutin in pear peels. Food Sci Biotechnol 25, 987–992 (2016). https://doi.org/10.1007/s10068-016-0160-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-016-0160-1