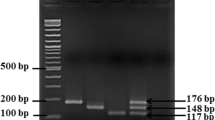
Abstract
Campylobacter is an important food-borne pathogen causing acute gastroenteritis worldwide. Magnetic nanoparticle-based PCR coupled with streptavidin-horseradish peroxidase and a substrate was used for colorimetric detection. Forward primers conjugated to magnetic nanoparticles facilitated separation and concentration of Campylobacter DNA in a sample matrix. After PCR, a green color developed and was observed using the unaided eye, or detected using a spectrophotometer. High specificity and sensitivity of the 100 fg DNA/PCR reaction were achieved in pure culture experiments. The technique was applied for detection of Campylobacter on naturally contaminated chicken skin. All positive results were in agreement with results achieved using a conventional culture method. The magnetic nanoparticle-PCR-enzyme linked gene assay was practical and useful for detection of Campylobacter in complex matrices with PCR-interfering substances.
Similar content being viewed by others
References
Butzler JP. Campylobacter from obscurity to celebrity. Clin. Microbiol. Infec. 10: 868–876 (2004)
Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J. Infect. Dis. 176: 92–98 (1997)
Hannu T, Mattlia L, Rautelin H, Pelkonen P, Lahdenne P, Siitonen A, Leirisalo-Repo M. Campylobacter-triggered reactive arthritis: A population-based study. Rheumatolog. 41: 312–318 (2002)
Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 5: 665–679 (2007)
Vindigni SM, Srijan A, Wongstitwilairoong B, Marcus R, Meek J, Riley PL, Mason C. Prevalence of foodborne microorganisms in retail foods in Thailand. Foodborne Pathog. Dis. 4: 208–215 (2007)
Suzuki H, Yamamoto S. Campylobacter contamination in retail poultry meats and by-products in the world: A literature survey. J. Vet. Med. Sci. 71: 255–261 (2009)
ISO 10272-1. Microbiology of food and animal feeding stuffs-Horizontal method for detection and numeration of Campylobacter spp.-Part 1: Detection method. International Standard Organization, Geneva, Switzerland (2006)
Bang DD, Pedersen K, Madsen M. Development of a PCR assay suitable for Campylobacter spp. mass screening programs in broiler production. J. Rapid Meth. Aut. Mic. 9: 97–113 (2001)
Bang DD, Wedderkopp A, Pedersen K, Madsen M. Rapid PCR using nested primers of the 16S rRNA and the hippuricase (hipO) genes to detect Campylobacter jejuni and Campylobacter coli in environmental samples. Mol. Cell. Probe. 16: 359–369 (2002)
Persson S, Petersen HM, Jespersgaard C, Olsen KEP. Realtime TaqMan polymerase chain reaction-based genus-identification and pyrosequencing based species identification of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus directly on stool samples. Diagn. Micr. Infec. Dis. 74: 6–10 (2012)
Federighi M, Tholozan J, Cappelier JM, Tissier JP, Jouve JL. Evidence of noncoccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scaning electron microscopy. Food Microbiol. 15: 539–550 (1998)
Adler M, Wacker R, Niemeyer CM. Sensitivity by combination: Immuno-PCR and related technologies. Analys. 133: 702–718 (2008)
Hong Y, Berrang ME, Liu T, Hofacre CL, Sanchez S, Wang L, Maurer JJ. Rapid detection of Campylobacter coli, C. jejuni, and Salmonella enterica on poultry carcasses by using PCR-enzyme-linked immunosorbent assay. Appl. Environ. Microb. 69: 3492–3499 (2003)
Safarik I, Safarikova M. Magnetic nano-and microparticles in biotechnology. Chem. Paper. 63: 497–505 (2009)
Yu LSL, Unkalis J, Tu SI. Immunomagnetic separation methods for the isolation of Campylobacter jejuni from ground poultry meats. J. Immunol. Method. 256: 11–18 (2001)
Morales-Rayas R, Wolffs PFG, Griffiths MW. Immunocapture and real-time PCR to detect Campylobacter spp. J. Food Protect. 71: 2543–2547 (2008)
Adedayo O, Kirkpatrick BD. Campylobacter jejuni infections: Update on presentation, diagnosis, and management. Hosp. Physicia. 44: 9–15 (2008)
Josefen MH, Lübek PS, Hansen F, Hoorfar J. Toward an international standard for PCR-based detection of foodborne thermotolerant campylobacters: Interaction of enrichment media and pre-PCR treatment on carcass rinse samples. J. Microbiol. Meth. 58: 39–48 (2004)
Walsh MK, Wang X, Weimer BC. Optimizing the immobilization of singlestranded DNA onto glass beads. J. Biochem. Bioph. Meth. 47: 221–231 (2001)
Li H, He Z. Magnetic bead-based DNA hybridization assay with chemiluminescence and chemiluminescent imaging detection. Analys. 134: 800–804 (2009)
Amagliani G, Omicciolio E, Del Campo, A, Bruce IJ, Brandi G, Magnani M. Development of a magnetic capture hybridization-PCR assay for Listeria monocytogenes direct detection in milk samples. J. Appl. Microbiol. 100: 375–383 (2006)
Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingwort T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrel BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Natur. 403: 665–668 (2000)
Jangpatarapongsa K, Polpanch D, Yamkamon V, Diththarot Y, Peng-On J, Thiramanas R, Hongseng S, Jootar S, Charoenmak L, Tangboriboonrat P. DNA detection of chronic myelogenous leukemia by magnetic nanoparticles. Analys. 136: 354–358 (2011)
Sails AD, Fox AJ, Bolton FJ, Wareing DRA, Greenway DLA, Borrow R. Development of a PCR ELISA assay for the identification of Campylobacter jejuni and Campylobacter coli. Mol. Cell. Probe. 15: 291–300 (2001)
Grennan B, O’Sullivan NA, Fallon R, Carroll C, Smith T, Glennon M, Maher M. PCR-ELISAs for the detection of Campylobacter jejuni and Campylobacter coli in poultry samples. BioTechnique. 30: 602–610 (2001)
Sails AD, Fox AJ, Bolton FJ, Wareing DRA, Greenway LA. A real-time PCR assay for the detection of Campylobacter jejuni in foods after enrichment culture. Appl. Environ. Microb. 69: 1383–1390 (2003)
Davis MA, Conner DE. Incidence of Campylobacter from raw, retail poultry products. Poultry Sci. 79(Suppl. 1): 54 (2000)
Davis MA, Conner DE. Survival of Campylobacter jejuni on poultry skin and meat at varying temperatures. Poultry Sci. 86: 765–767 (2007)
Sasaki Y, Haruna M, Mori T, Kusukawa M, Murakami M, Tsujuyama Y, Ito K, Toyofuku H, Yamada Y. Quantitative estimation of Campylobacter crosscontamination in carcasses and chicken products at an abattoir. Food Contro. 43: 10–17 (2014)
Al-soud WA, Jönsson LJ, Rådström P. Identification and characterization of immunoglobulin G in blood as a major inhibitor of diagnostic PCR. J. Clin. Microbiol. 38: 345–350 (2000)
Al-soud WA, Rådström P. Purification and characterization of PCR inhibitory components in blood cells. J. Clin. Microbiol. 39: 485–493 (2001)
Rossen L, Nórskov P, Holmstrøm K, Rasmussen OF. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 17: 37–45 (1992)
Thunberg RL, Tran TT, Walderhaung MO. Detection of thermophilic Campylobacter spp. in blood-free enriched samples of inoculated foods by the polymerase chain reaction. J. Food Protect. 63: 299–303 (2000)
Dickinson JH, Nkroll RG, Astori KA. The direct application of the polymerase chain reaction to DNA extracted from foods. Lett. Appl. Microbiol. 20: 212–216 (1995)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jansaento, W., Jangpatarapongsa, K., Polpanich, D. et al. Detection of Campylobacter DNA using magnetic nanoparticles coupled with PCR and a colorimetric end-point system. Food Sci Biotechnol 25, 193–198 (2016). https://doi.org/10.1007/s10068-016-0029-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-016-0029-3