Abstract
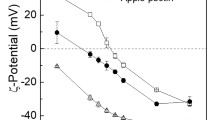
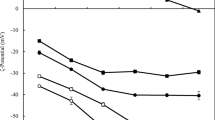
Electrostatic interactions within mixtures of a canola protein isolate (CPI) and both low (LMP) and high-methoxyl (HMP) pectin were investigated as a function of mixing ratio (1:1 to 30:1; CPI-pectin) and pH (8.0-1.5) using turbidity and electrophoretic mobility measurements during an acid titration. The rheological (flow behavior) and functional (solubility, foaming, and emulsifying properties) attributes of CPI-pectin complexes were also studied. Increasing biopolymer mixing ratios shifted critical pH values associated with formation of soluble and insoluble complexes to higher values until plateauing at approximately 10:1. Maximum coacervation of CPI-HMP and CPI-LMP mixtures occurred at pH values of 5.3 and 4.8, respectively, and at a 10:1 mixing ratio. The functionality of formed complexes was similar to CPI alone, except for a slight increase in solubility for the CPI-HMP system and a reduction in foaming capacity for CPI-LMP mixtures. For both mixed systems, viscosity was enhanced relative to CPI alone, showing greater pseudoplastic behavior.
Similar content being viewed by others
References
Tolstoguzov VB. Functional properties of food proteins and role of protein-polysaccharide interaction. Food Hydrocolloid. 4: 429–468 (1991)
Schmitt C, Sanchez C, Desobry-Banon S, Hardy J. Structure and technofunctional properties of protein-polysaccharide complexes: A review. Crit. Rev. Food Sci. 38: 689–753 (1998)
de Kruif CG, Weinbreck F, de Vries R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid In. 9: 340–349 (2004)
Li YJ, Xia JL, Dubin PL. Complex formation between polyelectrolyte and oppositely charged mixed micelles: Static and dynamic light scattering study of the effect of polyelectrolyte molecular weight and concentration. Macromolecules 27: 7049–7055 (1994)
Liu S, Low NH, Nickerson MT. Effect of pH, salt, and biopolymer ratio on the formation of pea protein isolate-gum arabic complexes. J. Agr. Food Chem. 57: 1521–1526 (2009)
Weinbreck F, Tromp RH, de Kruif CG. Composition and structure of whey protein/gum arabic coacervates. Biomacromolecules 5: 1437–1445 (2004)
Bohidar H, Dubin PL, Majhi PR, Tribet C, Jaeger W. Effects of protein-polyelectrolyte affinity and polyelectrolyte molecular weight on dynamic properties of bovine serum albumin-poly(diallyldimethylammonium chloride) coacervates. Biomacromolecules 6: 1573–1585 (2005)
Lizarraga MS, Vicin PD, Gonzalez R, Rubiolo A, Santiago LG. Rheological behaviour of whey protein concentrate and ëcarrageenan aqueous mixtures. Food Hydrocolloid. 20: 740–748 (2006)
Wang XY, Lee JY, Wang YW, Huang QR. Composition and rheological properties of beta-lactoglobulin/pectin coacervates: Effects of salt concentration and initial protein/polysaccharide ratio. Biomacromolecules 8: 992–997 (2007)
Ru Q, Wang Y, Lee J, Ding Y, Huang Q. Turbidity and rheological properties of bovine serum albumin/pectin coacervates: Effect of salt concentration and initial protein/polysaccharide ratio. Carbohyd. Polym. 88: 838–846 (2012)
Ye A. Complexation between milk proteins and polysaccharides via electrostatic interaction: Principles and applications-A review. Int. J. Food Sci. Tech. 43: 406–415 (2008)
Yuan Y, Wan Z-L, Yin S-W, Yang X-Q, Qi J-R, Liu G-Q, Zhang Y. Characterization of complexes of soy protein and chitosan heated at low pH. LWT-Food Sci. Technol. 50: 657–664 (2013)
Ortiz SE, Puppo MC, Wagner JR. Relationship between structural changes and functional properties of soy protein isolatescarrageenan systems. Food Hydrocolloid. 18: 1045–1053 (2004)
Plashchina IG, Mrachkovskaya TA, Danilenko AN, Kozhevnikov GO, Starodubrovskaya NY, Braudo EE, Schwenke KD. Complex formation of faba bean legumin with chitosan: Activity and emulsion properties of complexes. pp. 293–303. In: Food Colloids: Fundamentals of Formulation. Dickinson E, Miller R (eds). Royal Society of Chemistry, London, UK (2001)
Uruakpa FO, Arntfield SD. Emulsifying characteristics of commercial canola protein-hydrocolloid systems. Food Res. Int. 38: 659–672 (2005)
Li X, Fang Y, Al-Assaf S, Phillips GO, Jiang F. Complexation of bovine serum albumin and sugar beet pectin: Stabilizing oil-in-water emulsions. J. Colloid Interf. Sci. 388: 103–111 (2012)
Gu YS, Decker EA, McClements DJ. Influence of pH and carrageenan type on properties of ß-lactoglobulin stabilized oil-inwater emulsions. Food Hydrocolloid. 19: 83–91 (2005)
Ray M, Rousseau D. Stabilization of oil-in-water emulsions using mixtures of denatured soy whey proteins and soluble soybean polysaccharide. Food Res. Int. 52: 298–307 (2013)
Miquelim JN, Lannes SCS, Mezzenga R. pH influence on the stability of foams with protein-polysaccharide complexes at their interfaces. Food Hydrocolloid. 24: 398–405 (2010)
Schmidt I, Novales B, Boue F, Axelos MAV. Foaming properties of protein/pectin electrostatic complexes and foam structure at nanoscale. J Colloid Interf. Sci. 345: 316–324 (2010)
Lampart-Szczapa E. Legume and oilseed proteins. pp. 407–432. In: Chemical and functional properties of food proteins. Sikorski ZE (ed). CRC Press, Boca Raton, FL, USA (2001)
Bérot S, Compoint JP, Larré C, Malabat C, Guéguen J. Large scale purification of rapeseed proteins (Brassica napus L.). J. Chromatogr. B 818: 35–42 (2005)
Voragen AGJ, Pilnik W, Thibault JF, Axelos MAV, Renard CMGC. Pectins. pp. 287–339. In: Food Polysaccharides and Their Applications. Stephen AM (ed). Marcel Dekker Inc., New York, NY, USA (1995)
Klassen DR, Elmer CM, Nickerson MT. Associative phase separation involving canola protein isolate with both sulphated and carboxylated polysaccharides. Food Chem. 126: 1094–1101 (2011)
Folawiyo YL, Apenten RKO. Effect of pH and ionic strength on the heat stability of rapeseed 12S globulin (cruciferin) by the ANS fluorescence method. J. Sci. Food Agr. 70: 241–246 (1996)
AOAC. Official Method of Analysis of AOAC Intl. 17th ed. Method 925.10. Association of Official Analytical Chemists, Inc., Gaithersburg, MD, USA (2003)
AOAC. Official Method of Analysis of AOAC Intl. 17th ed. Method 923.03. Association of Official Analytical Chemists, Inc., Gaithersburg, MD, USA (2003)
AOAC. Official Method of Analysis of AOAC Intl. 17th ed. Method 920.87. Association of Official Analytical Chemists, Inc., Gaithersburg, MD, USA (2003)
AOAC. Official Method of Analysis of AOAC Intl. 17th ed. Method 920.85. Association of Official Analytical Chemists, Inc., Gaithersburg, MD, USA (2003)
Weinbreck F, de Vries R, Schrooyen P, de Kruif CG. Complex coacervation of whey proteins and gum arabic. Biomacromolecules 4: 293–303 (2003)
Morr CV, German B, Kinsella JE, Regenstein JM, Van Buren JP, Kilara A, Lewis BA, Mangino ME. A collaborative study to develop a standardized food protein solubility procedure. J. Food Sci. 50: 1715–1718 (1985)
Liu S, Elmer C, Low NH, Nickerson MT. Effect of pH on the functional behaviour of pea protein isolate-gum Arabic complexes. Food Res. Int. 43: 489–495 (2010)
Stone AK, Cheung L, Chang C, Nickerson MT. Formation and functionality of soluble and insoluble electrostatic complexes within mixtures of canola protein isolate and (Ϋ-, ι- and λ-type) carrageenan. Food Res. Int. 54: 195–202 (2013)
Liu S, Cao Y-L, Ghosh S, Rousseau D, Low NH, Nickerson MT. Intermolecular interaction during complex coacervation of pea protein isolate and gum arabic. J. Agr. Food Chem. 58: 552–556 (2010)
Klassen D R, Nickerson MT. Effect of pH on the formation of electrostatic complexes within admixtures of partially purified pea proteins (legumin and vicilin) and gum Arabic polysaccharides. Food Res. Int. 46: 167–176 (2012)
Aryee FNA, Nickerson MT. Formation of electrostatic complexes involving mixtures of lentil protein isolates and gum Arabic polysaccharides. Food Res. Int. 48: 520–527 (2012)
Girard M, Turgeon SL, Gauthier SF. Interbiopolymer complexing between beta-lactoglobulin and low- and high-methylated pectin measured by potentiometric titration and ultrafiltration. Food Hydrocolloid. 16: 585–591 (2002)
Sperber BLHM, Schols HA, Cohen Stuart MA, Norde WA, Voragen GJ. Influence of the overall charge and local charge density of pectin on the complex formation between pectin and betalactoglobulin. Food Hydrocolloid. 23: 765–772 (2009)
Lutz R, Aserin A, Portnoy Y, Gottlieb M, Garti N. On the confocal images and the rheology of whey protein isolated and modified pectins associated complex. Colloid. Surface. B 69: 43–50 (2009)
Burova TV, Grinberg NV, Grinberg VY, Usov AI, Tolstoguzov VB, de Kruif CG. Conformational changes in iota- and kappa-carrageenan induced by complex formation with bovine beta-casein. Biomacromolecules 8: 368–375 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stone, A.K., Teymurova, A., Chang, C. et al. Formation and functionality of canola protein isolate with both high- and low-methoxyl pectin under associative conditions. Food Sci Biotechnol 24, 1209–1218 (2015). https://doi.org/10.1007/s10068-015-0155-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-015-0155-3