Abstract
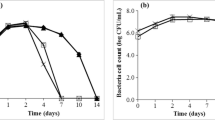
Preparation of Platycodi radix (PR) wine and assessment of biotransformation patterns of PR platycosides using co-cultures of Saccharomyces cerevisiae KCTC 7928 with Aspergillus awamori FMB S900 were performed. The basal fermentation temperature was 15°C and a high sucrose concentration (30% and above) was used to increase the level of ethanol production. Fermentation of PR in co-cultures of S. cerevisiae with A. awamori was compared to single culture fermentations using S. cerevisiae at concentrations of 30–50% (w/w) sucrose with analysis at an interval of 15 days during fermentation. The 3 major saponins (platycoside E, platycodin D3, and platycodin D) were converted into 16-oxo-PD during co-cultures of S. cerevisiae with A. awamori with production of up to 12.5% ethanol (with an initial sugar concentration of 40%).
Similar content being viewed by others
References
Ha IJ, Ha YW, Kang MS, Lee JS, Park DH, Kim YS. Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography. J. Sep. Sci. 33: 1916–1922 (2010)
Takagi K, Lee EB. Pharmacological studies on Platycodon grandiflorum A. DC. 3. Activities of crude platycodin on respiratory and circulatory systems and its other pharmacological activities. Yakugaku Zasshi. 92: 969–973 (1972)
Kim KS, Ezaki O, Ikemoto S, Itakura H. Effects of Platycodon grandiflorum feeding on serum and liver lipid concentrations in rats with diet-induced hyperlipidemia. J. Nutr. Sci. Vitaminol. 41: 485–491 (1995)
Kwon DY, Kim YS, Hong SM, Park SM. Long-term consumption of saponins derived from Platycodi radix (22 years old) enhances hepatic insulin sensitivity and glucose-stimulated insulin secretion in 90 % pancreatectomized diabetic rats fed a high-fat diet. Brit. J. Nutr. 101: 358–366 (2008)
Li W, Zhang W, Xiang L, Wang Z, Zheng YN, Wang YP, Zhang J, Chen L. Platycoside N: A new oleanane-type triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules 15: 8702–8708 (2010)
Park SJ, Lee HA, Kim JW, Lee BS, Kim EJ. Platycodon grandiflorus alleviates DNCB-induced atopy-like dermatitis in NC/Nga mice. Indian J. Pharmacol. 44: 469–474 (2012)
Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol. 93: 409–415 (2004)
Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-nB activation in RAW 264.7 cells. Life Sci. 76: 2315–2328 (2004)
Rao AV, Gurfinkel DM. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metabol. Drug Interact. 17: 211–235 (2000)
Na YC, Ha YW, Kim YS, Kim KJ. Structural analysis of platycosides in PR by liquid chromatography/electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 1189: 467–475 (2008)
Li W, Sun YS, Wang Z, Zheng YN. Isolation and purification of saponins from Platycodon grandiflorum by semi-preparative high performance liquid chromatography and LC/ESI-MS. J. Liq. Chromatogr. R. T. 35: 547–557 (2012)
Ha YW, Na YC, Seo JJ, Kim SN, Linhardt RJ, Kim YS. Qualitative and quantitative determination of ten major saponins in PR by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. J. Chromatogr. A 1135: 27–35 (2006)
Li W, Xiang L, Zhang J, Zheng YN, Han LK, Saito M. A new triterpenoid saponin from the roots of Platycodon grandiflorum. Chinese Chem. Lett. 18: 306–308 (2007)
Hawksworth G, Drasar BS, Hili MJ. Intestinal bacteria and the hydrolysis of glycosidic bonds. J. Med. Microbiol. 4: 451–459 (1971)
Sun Y, Cheng JY. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresource Technol. 83: 1–11 (2002)
Loughlin WA. Biotransformations in organic synthesis. Bioresource Technol. 74: 49–62 (2000)
Guang TC, Min Y, Yan S, Zhi QL, Jin QZ, Hui LH, Li JW, De AG. Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl. Microbiol. Biot. 77: 1345–1350 (2008)
Demirci A, Pometto AL. Enhanced organically bound chromium yeast production. J. Agr. Food Chem. 48: 531–536 (2000)
David CS, Thomas M. Woodxylanase product ion by Aspergillus awamori development of a medium and optimization of the fermentation parameters for the production of extracellular xylanase and p-xylosidase while maintaining low protease production. Biotechnol. Bioeng. 38: 883–890 (1991)
Asher MJ, Cowe IA, Thomas CE. Rapid method of counting spores of fungal pathogens by infra-red reflectance analysis. J. Plant Pathol. 31: 363–371 (1982)
Han LK, Xu BJ, Kimura Y, Zheng YN, Okuda H. PR affects lipid metabolism in mice with high fat diet-induced obesity. J. Nutr. 130: 2760–2764 (2000)
Wu JJ, Ma YK, Zhang FF, Chen FS. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “Shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 30: 289–297 (2012)
National Tax Service Technical Service Institute, Korea. Korea National Tax Service Liquor Analysis Regulation. National Tax Service Technical Service Institute, Seoul, Korea. pp. 62–66 (2008)
Turker N, Coskuner Y, Ekiz HI, Aksay S, Karababa E. The effects of fermentation on the thermostability of the yellow-orange pigments extracted from cactus pear (Opuntia ficus-indica). Eur. Food Res. Technol. 212: 213–216 (2001)
Montgomery R. Further studies of the phenol-sulfuric acid reagent for carbohydrates. Biochim. Biophys. Acta 48: 591–593 (1961)
Friedrich J, Cimerman A, Perdih A. Mixed culture of Aspergillus awamori and Trichoderma reesei for bioconversion of apple distillery waste. Appl. Microbiol. Biot. 26: 299–303 (1987)
Park YS, Ha DM. Isolation and identification of the lactic acid bacteria from nuruk. J. Korean Soc. Appl. Bi. 38: 95–99 (1995)
Park RD. Preparation of Korean Traditional Wine. Osang Press, Seoul, Korea. pp. 30–67 (2002)
Min YK, Jeong HS. Manufacture of some Korean medicinal herb liquors by soaking. Korean J. Food Sci. Technol. 27: 210–215 (1995)
Kim JH, Lee SH, Kim NM, Choi SY, Yoo JY, Lee JS. Manufacture and physiological functionality of Korean traditional liquor by using dandelion (Taraxecum platycarpum). Korean J. Appl. Microbiol. Biotechnol. 28: 367–371 (2000)
Lee DH, Kim JH, Kim NM, Choi JS, Lee JS. Physiological functionality of Chinese quince wine and liquors. Korean J. Biotechnol. Bioeng. 17: 266–270 (2002)
Holcberg IB, Margalith P. Alcoholic fermentation by immobilized yeast at high sugar concentrations. Eur. J. Appl. Microbiol. 13: 133–140 (1981)
Koppensteiner G, Windisch S. Osmotischer wert bei wachsturn und garung von hefen. Arch. Microbiol. 80: 300–314 (1971)
Torija MJ, Beltran G, Novo M, Poblet M, Guillamón JM, Mas A. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 85: 127–136 (2003)
Yoon YJ, Kim NY, Rhee YK, Han MJ. Quality characteristics and biological activities of traditionally fermented ginseng wine. Food Sci. Biotechnol. 16: 198–204 (2007)
Park WM, Park HG, Rhee SJ, Lee CH, Yoon KE. Suitability of domestic grape, cultivar Campbell’s early for production of red wine. Korean J. Food Sci. Technol. 34: 590–596 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, Y.l., Ahn, H.J. & Ji, G.E. Fermentation of Platycodi radix and bioconversion of platycosides using co-cultures of Saccharomyces cerevisiae KCTC 7928 and Aspergillus awamori FMB S900. Food Sci Biotechnol 24, 183–189 (2015). https://doi.org/10.1007/s10068-015-0025-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-015-0025-z