Abstract
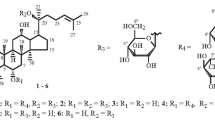
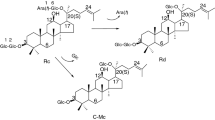
Preparative-scale fermentation of ginsenoside Rb1 (1) with Acremonium strictum AS 3.2058 gave three new compounds, 12β-hydroxydammar-3-one-20 (S)-O-β-d-glucopyranoside (7), 12β, 25-dihydroxydammar-(E)-20(22)-ene-3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (8), and 12β, 20 (R), 25-trihydroxydammar-3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (9), along with five known compounds, ginsenoside Rd (2), gypenoside XVII (3), ginsenoside Rg3 (4), ginsenoside F2 (5), and compound K (6). The structural elucidation of these metabolites was based primarily on one- and two-dimensional nuclear magnetic resonance and high-resolution electron spray ionization mass spectra analyses. Among these compounds, 2–6 are also the metabolites of ginsenoside Rb1 in mammals. This result demonstrated that microbial culture parallels mammalian metabolism; therefore, A. strictum might be a useful tool for generating mammalian metabolites of related analogs of ginsenosides for complete structural identification and for further use in pharmaceutical research in this series of compounds. In addition, the biotransformation kinetics was also investigated.


Similar content being viewed by others
References
Dou DQ, Chen YJ, Liang LH, Pang FG, Shimizu N, Takeda T (2001) Six new dammarane-type triterpene saponins from the leaves of Panax ginseng. Chem Pharm Bull 49:442–446
Li L, Liu RX, Ye M, Hu XY, Wang Q, Bi KS, Guo DA (2006a) Microbial metabolism of evodiamine by Penicillium janthinellum and its application for metabolite identification in rat urine. Enzyme Microb Technol 39:561–567
Li L, Ye M, Bi KS, Guo DA (2006b) Liquid chromatography–tandem mass spectrometry for the identification of L-tetrahydropalmatine metabolites in Penicillium janthinellum and rats. Biomed Chromatogr 20:95–100
Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M (1997) Protection of ischemic hippocamal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res 28:191–200
Longhlin WA (2000) Biotransformation in organic synthesis. Biores Technol 74:49–62
Ma WG, Mjizutani M, Malterud KE, Lu SL, Ducrey B, Tahara S (1999) Saponins from the roots of Panax notoginseng. Phytochemistry 52:1133–1139
Ma XC, Ye M, Wu LJ, Guo DA (2006) Microbial transformation of curdione by Mucor spinosus. Enzyme Microb Technol 38:367–371
Ma XC, Zheng J, Guo DA (2007) Highly selective isomerization and dehydrogenation of three major bufadienolides at 3-OH by Fusarium solani. Enzyme Microb Technol 40:1585–1591
Odani T, Tanizawa H, Takino Y (1983) Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. III. The absorption, distribution and excretion of ginsenoside Rb1 in the rat. Chem Pharm Bull 31:1059–1066
Qian TX, Jiang ZH, Cai ZW (2006) High-performance liquid chromatography coupled with tandem mass spectrometry applied for metabolic study of ginsenoside Rb1 on rat. Anal Biochem 352:87–96
Rao GP, Davis PJ (1997) Microbial models of mammalian metabolism. Biotransformation of HP 749 (Besipirdine) using Cunninghamella elegans. Drug Metab Dispos 25:709–715
Smith RV, Rosazza JP (1974) Microbial models of mammalian metabolism, aromatic hydroxylation. Arch Biochem Biophys 161:551–558
Smith RV, Rosazza JP (1975) Microbial models of mammalian metabolism. J Pharm Sci 11:1737–1758
Takina Y (1994) Studies on the pharmacodynamics of ginsenoside Rg1, ginsenoside Rb1 and ginsenoside Rb2 in rats. Yakugaku Zasshi 114:550–564
Teng RW, Li HZ, Wang DZ, He YN, Yang CR (2000) NMR complete assignments of three protopanaxadiol mondesmosides. Chin J Magn Reson 17:461–468
Teng RW, Li HZ, Wang DZ, He YN, Yang CR (2002) NMR complete assignments of three protopanaxadiol bisdesmosides. Chin J Magn Reson 19:25–32
Wang XY, Zhang JT (2003) Effect of ginsenoside Rb1 on long-term potentiation in the dentate gyrus of anaesthetized rats. J Asian Nat Prod Res 5:1–4
Ye M, Qu G, Guo H, Guo DA (2004) Specific 12β-hydroxylation of cinobufagin by filamentous fungi. Appl Environ Microbiol 70:3521–3527
Yoshikawa M, Morikawa T, Kashima Y, Ninomiya K, Matsuda H (2003) Structure of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins. J Nat Prod 66:922–927
Zhao P, Liu YQ, Yang CR (1996) Minor dammarane saponins from Panax notoginseng. Phytochemistry 41:1419–1422
Acknowledgment
This work was supported by Shanghai Commission of Science and Technology (0511021024) and National Supporting Program for Traditional Chinese Medicine from the Ministry of Science and Technology of China (2006BAI08B03-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, GT., Yang, M., Song, Y. et al. Microbial transformation of ginsenoside Rb1 by Acremonium strictum . Appl Microbiol Biotechnol 77, 1345–1350 (2008). https://doi.org/10.1007/s00253-007-1258-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1258-4