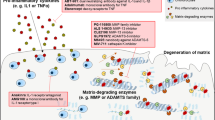
Abstract
The burden of osteoarthritis (OA) is rapidly increasing with population aging, but there are still no approved disease-modifying drugs available. Accumulating evidence has shown that OA is a heterogeneous disease with multiple phenotypes, and it is unlikely to respond to one-size-fits-all treatments. Inflammation is recognized as an important phenotype of OA and is associated with worse pain and joint deterioration. Therefore, it is believed that anti-inflammatory treatments may be more effective for OA with an inflammatory phenotype. In this review, we summarized clinical trials that evaluated anti-inflammatory treatments for OA and discussed whether these treatments are more effective in inflammatory OA phenotypes compared to general OA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common joint disease affecting approximately 240 million people worldwide [1]. The number continues to increase with population aging and rising obesity rates, making OA an unignorable public health problem. However, no approved treatments are available to prevent or even retard OA disease progression [2]. Current treatments focus on relieving symptoms but the efficacy and safety of analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) are often not desirable [3], especially in the long-term [4].
Accumulating evidence has shown that OA is a heterogeneous disease with multiple phenotypes [5, 6], and defining OA phenotypes is commonly based on characteristics of joint tissues (e.g., bone, cartilage, synovial tissue) and clinical assessments (e.g., comorbidity, symptoms, biochemical tests). Previous studies have summarized potential OA phenotypes such as bone phenotype, metabolic syndrome phenotype, pain phenotype, and inflammatory phenotype [6, 7]. These phenotypes are largely different in many aspects, making one-size-fits-all treatments unlikely. Among these OA phenotypes, inflammatory OA is a common type (prevalence approximately 16% to 60% in different samples [7, 8]) and is usually characterized by overexpression of inflammatory cytokines, and the increased signal intensity on ultrasound (US) or magnetic resonance imaging (MRI) that results from inflammation of the joint lining (synovitis) and joint fluid (effusion), termed “effusion-synovitis”. Epidemiological studies have shown that effusion-synovitis may be a source of OA symptoms and predict both symptomatic and structural progression of OA [9]. Therefore, it is believed that treatments targeting inflammation can delay or prevent cartilage damage and osteophyte formation. This suggests that inflammation is a therapeutic target for the inflammatory phenotype of OA and that anti-inflammatory treatments are more likely to achieve desirable outcomes. In this review, we summarized clinical trials that evaluated anti-inflammatory treatments for OA and discussed whether these treatments are more effective in inflammatory OA phenotypes compared to general OA patients.
Literature research strategy
We adopted a hybrid search strategy that included both database searches and snowballing. We searched PubMed and Web of Science from inception to December 2023 using search terms including “OA”, “osteoarthritis”, “inflammation”, “synovitis”, “effusion”, “therapy”, and “treatment” for clinical trials. The bibliography of the included studies was also checked for additional studies. Two reviewers (RZ and HF) independently reviewed the identified articles and conducted risk of bias assessment according to the Cochrane Collaboration's tool for eligible trials [10], with disagreement discussed with a third author (GC). Clinical trials evaluating the effect of anti-inflammatory treatments for OA with or without an indicator of inflammation were included. We excluded reviews, animal and experimental studies, and observational studies. After study selection, 58 articles related to this review were included (Fig. 1, Tables 1, 2, and 3). Overall, 65% trials showed a low risk of bias (Supplementary Table 1).
Disease-modifying antirheumatic drugs (DMARDs)
Methotrexate
Methotrexate (MTX) is one of the first-line disease-modifying antirheumatic drugs (DMARDs) commonly used to treat synovitis in inflammatory arthritis, especially rheumatoid arthritis (RA), with satisfactory effectiveness and long-term safety [69]. The mechanisms of action of the anti-synovitis effect of methotrexate have been well documented [70]. It is hypothesized that MTX could also reduce synovitis and pain in inflammatory OA. Erosive hand OA is considered a form of inflammatory OA with poorer clinical and radiographic outcomes than non-erosive hand OA [71]. In a small open-label, single-arm study of 21 patients with erosive hand OA (published as a conference abstract only), the authors found that a weekly dose of 10 mg MTX decreased pain, stiffness, and functional disability, although number of tender and swollen joints did not change[11]. Similar findings were observed in a randomized controlled trial (RCT) of 64 patients with erosive hand OA showing that the same dose of MTX reduced visual analogue scale (VAS, 0–100, a higher score indicates worse pain) pain within the treatment group [12]. The reduction in pain was greater (albeit not statistically significant) in patients receiving MTX compared to placebo over 3 (-21.1 vs -11.7) but not 12 months (-14.3 vs -17.8) [12]. Neither trial limited their participants to having synovitis, and the later trial reported a very low prevalence of synovitis (29 of 1024 joints), which may have underestimated the effect of MTX [12]. In a recent RCT of 97 patients who had hand OA and MRI-detected synovitis, 20 mg of MTX per week showed a moderate and potentially clinically meaningful effect on relieving VAS pain and the Australian Canadian Osteoarthritis Hand Index (AUSCAN) stiffness but not function over a 6-month period compared to placebo [13]. The effect of MTX on radiographic progression and tender swollen joint count over 2 years was not able to be evaluated due to the COVID-19 pandemic [13].
For knee OA, a 6-month RCT showed that a weekly dose of 15 mg MTX reduced pain severity, functional status, and quality of life over 3 and 6 months in 100 patients with moderate to severe (KL score 3–4) knee OA compared to placebo[14]. However, another 4-month RCT did not observe any differences in change in pain and functional limitation between the 7.5 mg MTX and placebo groups, although there was a tendency to an increased consumption of paracetamol in the placebo group [15]. Preliminary findings from a multicenter RCT indicated that high dose of MTX (maximum 25 mg) improved 0–10 Numeric Rating Scale (NRS) knee pain (adjusted treatment difference -0.83 (95% CI -1.55 to -0.10), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness and function, but not WOMAC pain at 6 months compared to placebo (published in a conference abstract only) [16, 72]. While the effect on pain was moderate, it was below a clinically meaningful threshold, did not persist at 12 months and there were no changes in synovial volume [16]. The participants in this trial had clinical and radiographic knee OA but were not selected based on inflammation[72]. The variation in therapeutic efficacy of MTX across these studies may be attributed to differences in dosage. In an open-label, single-arm trial of 30 patients with symptomatic knee OA and effusion synovitis on ultrasound, 43% of patients achieved ≥ 30% reduction in knee pain after MTX treatments (20 mg weekly) over 24 weeks but there was no correlation between change in knee pain and change in synovitis thickness [17]. Given this was an open-label study with only one treatment group, potential placebo effects may have been overlooked. Although no formal RCTs have evaluated the effect of MTX on the structural progression of OA, current studies suggested that MTX can relieve joint symptoms and that a stronger effect in patients with synovitis is likely but needs to be confirmed. Several ongoing RCTs conducted in OA patients with synovitis will answer these questions [18, 19, 72]. High-dose MTX demonstrates promising analgesic effects in both hand and knee OA, with a good safety profile when used in conjunction with folic acid supplementation. Additionally, it may yield greater efficacy in patients with hand OA accompanied by synovitis, but it is still unclear in knee OA.
Hydroxychloroquine
Hydroxychloroquine (HCQ), an anti-malarial drug, is widely used in RA and systemic lupus erythematosus [73]. The therapeutic effect of HCQ may be related to its anti-inflammatory activity, including the inhibition of T-cell activation and cytokine release [74]. Increasing evidence suggests that these pathways may also be involved in OA [75], supporting HCQ as a potential treatment for inflammatory OA.
For hand OA, current evidence consistently suggests a lack of efficacy of HCQ on hand pain. In a recent RCT of 196 patients with symptomatic hand OA, pain relief was similar in the HCQ and placebo groups over 24 weeks [20]. Another RCT involving 153 patients with inflammatory (based on clinical symptoms) and erosive hand OA also showed that HCQ was not different from placebo in symptom relief [21]. Consistently, an RCT evaluating the efficacy of HCQ in erosive hand OA similarly demonstrated that 400 mg of HCQ for 30 days, followed by a reduction in dosage to 200 mg for 11 months, was not effective on VAS pain or Dreiser’s score [22]. It is worth noting that patients in the HCQ group reported poorer compliance, which could also influence the study results. In an RCT of 248 patients with hand OA and most of them had synovitis (134 of 143) in at least one joint by ultrasound, HCQ (200 to 400 mg of dosing according to weight) was no better than placebo for pain relief after 12 months [23]. No dose–response relationship was found for the efficacy, and there was no correlation observed between the presence of synovitis detected by power Doppler and the magnitude of the treatment effect.
For knee OA, the findings from two RCTs are inconsistent regarding the effect of HCQ on knee symptoms. In a small RCT of 29 patients with symptomatic and radiographic knee OA, 400 mg of HCQ per day for 4 months did not improve knee symptoms as assessed by WOMAC, Lequesne index, and VAS, compared to placebo [24]. In another RCT of 44 patients with knee pain and radiographic knee OA, however, 200 mg of HCQ twice per day significantly improved WOMAC pain (-12.24 VS -0.91, range 0–50), function (-29.90 VS -1.56, range 0–170), and stiffness (-2.24 VS -0.74, range 0–20) over 24 weeks compared to placebo [25]. In addition, consumption of painkillers was significantly higher in the placebo group than in the HCQ group.
In an RCT of 166 patients with moderate to severe knee OA and synovitis, detected clinically and by ultrasound, 400 mg of HCQ per day for 9 months reduced both VAS knee pain and synovitis compared to the placebo group (published as a conference abstract only) [26]. These studies imply that efficiency of HCQ in knee OA is inconsistent and thus further high-quality evidence is needed. In a recent meta-analysis of 6 RCTs of patients with knee or hand OA, pooled results showed no effect of HCQ on pain and function of hand or knee OA [76].
Biologic agents
Tumor necrosis factor-α (TNF-α) inhibitors
TNF-α is a proinflammatory cytokine that can be produced by a variety of cells including synovial cells and chondrocytes in OA [77]. In addition, TNF-α can directly stimulate osteoclast differentiation and enhance the expression of a series of proinflammatory cytokines [78]. Previous studies have shown that TNF-α is associated with OA pain [79]. TNF-α inhibitors, such as infliximab, adalimumab, and etanercept, suppress the immune system and inflammation by blocking the activity of TNF-α [80].
In a small pilot study of 10 women with erosive hand OA, 0.2 mg of infliximab per month improved both joint symptoms and disease progression after 1 year compared to saline (placebo) [27]. However, in an RCT of 85 patients with painful hand OA refractory to analgesics and NSAIDs, two subcutaneous injections of 40 mg adalimumab at a 15-day interval were not effective for pain relief after 6 weeks and 6 months, compared to placebo [28]. While this study did not restrict the participants to have synovitis, another crossover trial of 43 participants with erosive hand OA and MRI-detected synovitis showed similar results, that 40 mg of adalimumab (injections every other week) did not improve hand pain or synovitis after 12 weeks [29]. Moreover, in an RCT of 90 patients with erosive OA, 50 mg per day of etanercept for 24 weeks thereafter 25mg per day did not demonstrate significant pain relief after 1 year compared to placebo [30]. However, in prespecified per-protocol analyses, etanercept improved hand pain in patients who complied with the treatment and had pain and soft swelling, power Doppler signal, or MRI-detected synovitis at baseline [30]. Moreover, etanercept treatment was associated with a higher rate of radiographic remodeling and fewer bone marrow lesions, especially in joints with inflammation at baseline [30]. Consistently, another RCT of 60 patients with erosive hand OA also found that 40 mg of adalimumab every 2 weeks slowed erosive progression after 1 year [31].
For knee OA, except for a case report showing that adalimumab improved knee pain, synovitis, and bone marrow lesions after 6 months [81], there was only one open-label RCT including 56 knee OA patients assessing the efficacy of adalimumab compared with hyaluronic acid in moderate to severe knee OA [32]. Patients who received 10 mg of adalimumab for 4 weeks significantly improved VAS scores, WOMAC scores, Global Assessment (PGA), and Physician Global Assessment (PhGA) compared to those who received 25 mg of hyaluronic acid [32].
The findings from these RCTs suggest that TNF-α inhibitors may exhibit a therapeutic effect on both joint symptom and structural progression of hand OA over the long term (e.g. 1 year), especially in patients with a high level of inflammation. However, this needs to be confirmed in larger and longer RCTs. Low-level evidence suggests that TNF-α inhibitors may be effective for knee OA, but it is unclear whether the effect is stronger in patients with synovitis and there were no rigorously conducted RCTs to date.
Interleukin-1 inhibitors
Interleukin-1α (IL-1α) and IL-1β are proinflammatory cytokines that have similar effects as TNF-α in inflammatory arthritis, including involvement in structural damage and symptoms [82]. However, the performance of IL-1 inhibitors in RCTs for OA has been unsatisfactory. In an RCT of 228 patients with knee OA, AMG 108, an inhibitor of both IL-1α and IL-1β, did not significantly improve WOMAC pain scores (range 0–500) after 12 weeks compared to placebo (median change, -63 vs -37, p = 0.25) [33].
Another RCT including 170 patients with symptomatic knee OA showed similar results that intra-articular injection of anakinra (an IL-1 receptor antagonist), either 50 or 150 mg, had no effect on knee symptoms compared to placebo [34], and no significant improvement was found in cartilage degradation markers. In vitro studies have shown that at least 10–100 times excessive doses are required to affect the activity of IL-1β in cartilage [83]. It is important to note that neither trial, which evaluated the effect of anti-inflammatory treatments, restricted their participants to those with an OA inflammatory phenotype. Two RCTs sought to overcome this limitation by recruiting participants with OA and synovitis [35, 37]. One of them was a phase 2 trial that investigated the effect of lutikizumab on knee symptoms in 350 patients with radiographic knee OA and synovitis detected on ultrasound or MRI [35]. Lutikizumab is a novel human dual variable domain immunoglobulin that has shown better performance in pain and cartilage damage, compared to inhibition of IL-1α or IL-1β alone in a mouse model of OA [84]. In the phase 2 trial, however, lutikizumab of different doses (25, 100, or 200 mg subcutaneously every 2 weeks) did not show satisfactory effect on pain and synovitis (as measured by synovial membrane thickness, synovial fluid volume, and WORMS synovitis/effusion score) compared with placebo, only the 100 mg group showed a significant improvement in WOMAC pain at 16 weeks compared with placebo (P = 0.050) [35]. However, subsequent analysis revealed that patients with K/L grade 3 knee osteoarthritis continued to experience superior pain relief compared to placebo from 16 to 52 weeks. This suggests that patients with severe knee OA may derive greater benefit from treatment with lutikizumab. Meanwhile, in a phase I trial of patients with knee OA, lutikizumab treatment was associated with a reduction in serum levels of inflammatory biomarkers [36]. However, the decrease in the levels of inflammatory biomarkers did not result in additional therapeutic benefits. The inconsistent results between animal studies and human trials were thought to be due to different disease stages, severity of synovitis, and route of administrating IL-1 inhibitors [85]. A trial is underway to evaluate the effect of diacerein on knee pain and effusion-synovitis in patients with symptomatic knee OA and MRI-detected effusion-synovitis [37]. Diacerein is an oral IL-1β inhibitor which has been shown to be beneficial for pain and joint space narrowing (JSN) in OA patients, according to a systematic review of clinical trials [86]. Interestingly, in a post-hoc analysis of a large RCT (n = 10,061), canakinumab, an IL-1β inhibitor, reduced the risk of total knee and hip replacement surgeries and OA-related adverse events over 3.7 years, in participants with or without OA at baseline [38]. Previous studies have also indicated that IL-1β may play a role in driving cartilage degradation [87]. These findings suggest that IL-1 inhibitors may exhibit a potential role in the structural progression of OA.
Little is known about the effect of IL-1 inhibitors on hand OA. In an RCT of 132 patients with erosive hand OA and moderate to severe inflammation, confirmed by tender and/or swollen interphalangeal and synovitis by MRI at baseline, lutikizumab (200 mg every two weeks) did not improve the AUSCAN pain score or any imaging outcomes (e.g. JSN, osteophytes, synovitis, BMLs) after 26 weeks [39]. This is despite seeing a decrease in serum high-sensitivity C reactive protein levels, and IL-1α and IL-β levels [39], suggesting that even though lutikizumab affects inflammatory biomarkers this is not resulting in pain relief for patients.
The lack of desired pain relief with IL-1 inhibitors may be attributed to the possibility that, although synovium is a significant source of IL-1, IL-1 itself may not be essential for sustaining synovial inflammation. Exploring and discovering other factors such as cartilage degradation products and adipokines[9] associated with synovial inflammation may yield greater benefits.
Other treatments
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
The effect of both oral and topical Nonsteroidal Anti-inflammatory Drugs (NSAIDs) on symptomatic OA has been well documented in RCTs, although there are differences in effectiveness and safety among various NSAIDs [88]. In clinical practice, NSAIDs are widely used in OA patients to relieve joint symptoms, and they are recommended by the most up-to-date guidelines for the treatment of OA [89, 90].
NSAIDs alleviate pain and inflammation by inhibiting the synthesis of prostaglandins [91]. Both animal studies and in vitro experiments show a potential chondroprotective effect of NSAIDs [92, 93]. However, there is a scarce amount of RCTs to evaluate whether the effect of NSAIDs is more pronounced in patients with inflammatory OA. In an open-label trial of 90 patients scheduled for a total knee arthroplasty, high dosages of 3 common NSAIDs (i.e. celecoxib, diclofenac, and ibuprofen), compared to low dosages, significantly improved WOMAC score and decreased the concentration of inflammatory cytokines in the synovial fluid 14 days after beginning of treatment, suggesting a short-term effect of NSAIDs on both OA symptoms and inflammation [40]. In a placebo-controlled trial of 201 patients with knee pain, radiographic OA, and joint inflammation (documented by knee swelling), 2400 mg/day of ibuprofen prevented the increase of markers reflecting cartilage and synovium metabolism after 4 to 6 weeks of treatment, compared to placebo [41]. However, the effect on inflammatory markers does not necessarily translate to improvements in joint structures. Using data from 990 participants with moderate to severe knee OA in the Osteoarthritis Initiative, Luitjens et al. found no beneficial effect of NSAIDs on change in synovitis or cartilage thickness or composition over 4 years [94]. Moreover, in another study of 99 patients with erosive hand OA, cessation of NSAIDs over 2 weeks did not significantly change inflammatory features (i.e. synovial proliferation, effusion and power Doppler signal) on ultrasound compared with patients who did not regularly take NSAIDs [42]. As one of the most used anti-inflammatory treatments for OA, NSAIDs have well-known adverse effects affecting the gastric mucosa, renal system, cardiovascular system, hepatic system, and hematologic system [95]. Further studies are warranted to understand whether the effect of NSAIDs differs among patients with inflammatory OA and those without, thereby optimizing the use of NSAIDs in OA treatment.
Colchicine
Colchicine is an anti-inflammation drug often used to treat gout, which has the potential to prevent uric acid crystal-induced inflammation that exists in OA [96].
For hand OA, an RCT of 59 patients aged 40–80 years showed that 0.5 mg of colchicine twice per day was not effective on pain relief, tender and swollen joint count, or grip strength and synovitis grade compared to placebo for 12 weeks [43]. Similar results were observed in a recent RCT of 100 symptomatic hand OA patients, in which 0.5 mg of colchicine twice per day was not more effective on pain relief compared to placebo for 12 weeks, and the treatment led to more adverse events [44].
For knee OA, two pilot RCTs from the same group published in 2002 (n = 36 and 39, respectively) showed that colchicine added to nimesulide or intraarticular steroid improved VAS knee pain and WOMAC symptoms over 20 weeks compared to nimesulide or intraarticular steroid alone [45, 46]. These encouraging findings were repeated by one follow-up trial but not another.
An RCT of 61 postmenopausal patients with primary knee OA suggested that 0.5 mg of colchicine twice per day for 3 months can significant improved VAS pain score compared to placebo [47]. Another RCT of 109 symptomatic knee OA patients indicated that 0.5 mg of colchicine twice per day for 16 weeks was not effective on WOMAC scores although the mean levels of serum high-sensitivity C-reactive protein (hs-CRP) and synovial fluid C-terminal crosslinked telopeptide of Type I collagen were decreased compared with placebo [48]. The study quality of available evidence was low overall, and no RCT has specified the patients to have a sign of inflammation, for whom the anti-inflammatory property of colchicine may bring greater benefits.
Corticosteroids
Intra-articular corticosteroids are often used to improve the symptoms in OA patients [97]. However, the role of corticosteroids remains unclear in OA. One possible explanation is that corticosteroids may achieve analgesic purposes by inhibiting synovial inflammation [98]. In a trial evaluating the effect of a combination of prednisolone and dipyridamole, CRx-102 (comprises dipyridamole and low dose prednisolone) reduced AUSCAN pain scores in 83 hand OA patients[49]. Meanwhile, a previous study has shown that 120 mg of methylprednisolone intramuscular injection was effective on inflammatory hand pain [99]. At baseline, 55% of patients had ultrasound-detected synovitis and 23% had clinical synovitis. Interestingly, further analysis showed that ultrasound-detected synovitis was associated with treatment response, but not clinical synovitis. This seems to imply that methylprednisolone is more effective in patients with early synovitis.In an RCT of 70 participants with hand OA and 75% had MRI-detected synovitis, 5 mg of oral prednisolone per day was not effective in relieving VAS pain compared with placebo for 4 weeks [50]. The lack of analgesic effect may be due to the low dose. Another RCT included 92 patients with symptomatic hand OA and ultrasound-detected synovitis, and 10 mg of oral prednisolone daily significantly improved VAS hand pain compared to placebo (-21.5 vs -5.2). Moreover, synovitis thickening (by ultrasound) and BMLs but not soft tissue swelling or synovitis on MRI were also improved in the prednisolone group [51]. An Open-label study of 36 hand OA patients showed that 120 mg of intra-muscular methylprednisolone improved VAS pain and clinical symptoms but not grey-scale synovitis over 12 months [52]. This may be due to the fact that in the participants of this study, gray-scale synovitis on ultrasound was usually of low grade, and short-term treatment effects may be easily overlooked. Further analysis showed that patients who met the criteria for an Osteoarthritis Research Society International response criteria had more gray-scale synovitis and power Doppler signals at baseline. These findings suggest that corticosteroids may have a greater effect in inflammatory hand OA.
For knee OA, the role of corticosteroids in knee OA with synovitis is unclear. There was an RCT showing that intra-articular triamcinolone did not reduce knee pain and even resulted in more cartilage loss over 2 years in patients with symptomatic knee OA [100].
Fish oil and Krill oil
Fish oil and krill oil are rich in omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [101]. Omega-3 fatty acids can compete with omega-6 fatty acids to reduce pain, swelling, neutrophil, and metalloproteinase activation, thereby alleviating synovitis and delaying the progression of cartilage degeneration [102]. There is evidence that these supplements may be beneficial for synovitis and cartilage damage [54, 101].
Fish oil has been widely used in the combined treatment of RA. A literature review suggested that fish oil can relieve pain and morning stiffness and reduce the intake of non-steroid anti-inflammatory drugs in patients with RA [103]. Fish oil has also been used for OA with some efficacy [54, 104]. In an RCT of 152 overweight/obese older adults with OA pain (site not specified) measured by the short-form McGill Pain Questionnaire, fish oil reduced OA-specific pain and burden of OA over 16 weeks [53]. A separate study which evaluated high-dose fish oil compared to low-dose fish oil found that pain improved in both groups but that the low-dose fish oil group demonstrated the greatest improvement in WOMAC pain and function scores at 2 years [54]. There was no difference between the groups in cartilage volume loss or CRP levels over 2 years [54]. There were no studies to evaluate the effect of fish oil in OA patients with synovitis. Further studies are needed to confirm the role of fish oil in OA patients with synovitis.
Krill oil is also rich in EPA and DHA, and it contains antioxidants such as astaxanthin [101]. Krill oil had better effects than fish oil in experimental RA models [105]. There were 2 small RCTs (90 and 50 participants, respectively) indicating that krill oil can relieve pain, function, and inflammation in patients with knee OA [55, 56]. Another RCT involving 235 patients with OA showed that 4 g daily of krill oil (0.60 g EPA, 0.28 g DHA and 0.45 mg astaxanthin) provided modest pain relief over 6 months in patients with moderate to severe knee OA [57]. While these studies suggested that krill oil may be moderately effective on knee symptoms in general OA patients, a recent RCT of 262 patients with knee OA and synovitis showed no effect of 2 g of krill oil (0.19 g EPA and 0.1 g DHA) on pain or synovitis over 24 weeks [58]. Although the difference in efficacy between the two studies could be attributed to the dose, neither study reported any improvement in local inflammation. This suggests that the analgesic effect of krill oil, if any, may not be through its anti-inflammatory effect.
Curcuma longa extract
Curcuma longa extract (CL) is frequently used in traditional Chinese medicine and Ayurveda for the treatment of OA through its anti-inflammatory, antioxidant, and analgesic properties [106]. In an RCT of 160 patients with knee OA, 500 mg of CL extract per day suppressed biomarkers of both inflammation and oxidative stress and reduced knee pain compared to placebo [59]. The lack of improvement in radiographic manifestations of the joints after four months of treatment suggests that the alleviation of pain with CL may be attributed to reduced inflammation. This implies that CL extract may be more effective in patients with an inflammatory phenotype of OA. A systematic review of RCTs has shown that CL extract is beneficial for pain relief in patients with knee OA [107], although these trials did not include patients with inflammatory OA. In a recent RCT of 70 patients with symptomatic knee OA and ultrasound-defined effusion-synovitis, 2 capsules (2*500 mg) of CL extract significantly improved knee pain after 16 weeks [60]. However, unexpectedly, pain relief was only observed in patients with smaller but not larger effusion-synovitis at baseline. This contrasts with the hypothesis that CL extract may be more effective in patients with an inflammatory phenotype of OA. This may be due to that those with a higher effusion-synovitis volume had more severe disease, for which CL extract was less effective. Despite inconsistent dosages and slight variations in study design (regarding co-administration with nonsteroidal anti-inflammatory drugs), the current findings suggest that CL is moderately effective and safe for knee osteoarthritis. However, its potential to target synovitis for OA treatment remains ambiguous.
Chondroitin sulfate and glucosamine sulfate
Chondroitin sulfate (CS) and glucosamine sulfate (GS) are a glycosaminoglycans that has long been found to have anti-inflammatory and cartilage-protective effects in OA [108]. Animal and clinical studies suggested that CS can inhibit the progression of synovitis [109, 110]. A previous meta-analysis of RCTs has indicated that CS may be superior to placebo in pain relief for hip, knee and hand OA patients [111]. In an RCT of 353 patients with knee OA showed that both 1200 mg once daily and 400 mg three daily of CS was more effective in OA clinical symptoms relief measured by Lequesne index compared to placebo [61]. Another RCT of 69 patients with knee OA and clinical signs of synovitis (warmth, swelling, or effusion), CS 800 mg for 6 months significantly reduced cartilage volume loss at 6 months and bone marrow lesions at 12 months, compared to placebo [62].
The effect of GS in OA is controversial. Several RCTs showed that GS may be no effect in pain relief for hip and knee OA [63, 64]. In contrast, another RCT of 205 patients with knee OA showed that 1500 mg of GS daily was more effective in WOMAC pain, stiffness and physical function score for knee OA compared to placebo [65]. Compared to this study, the two negative studies did not exclude other rheumatic diseases, which could potentially influence the source of joint pain [63, 64]. Furthermore, one study suggests that variations in the formulation of GS may contribute to differences in efficacy [112], which could partially account for discrepancies in therapeutic outcomes. In addition, CS is often used in combination with GS [113]. But the results are controversial similarly. In an RCT of 164 patients with knee OA and moderate to severe pain, CS 1200 mg plus GS 1500 mg daily did not reduce VAS pain over 6 months [66]. Similarly, another large RCT that used the same dose showed that neither CS nor GS or their combination reached a greater proportion of pain relief by 20% compared to placebo over 24 weeks in 1583 patients with moderate-to-severe knee pain [67]. This is despite that another RCT involving 605 patients with knee OA showed that the CS 400 mg plus GS 753 mg significantly reduced JSN but not pain over 24 months [68]. As patients with synovitis tend to have higher levels of pain, a combination of CS and GS may achieve better outcomes in patients with synovitis. Interestingly, although studies of CS and GS have been mostly positive, neither of these treatments is recommended for the treatment of OA due to potential publication bias[114].
Conclusion
Inflammatory OA is a common and important phenotype of OA that may benefit more from anti-inflammatory treatments but to date there are limited high-quality RCTs which have selected OA patients based on inflammation. Current evidence suggests that MTX, TNF-α inhibitors and prednisolone may have a better efficacy on pain relief in patients with hand OA and synovitis. However, it is uncertain whether anti-inflammatory treatments have a stronger effect in pain relief for patients with knee OA and an inflammatory phenotype.
Data availability
Not applicable.
References
Katz JN, Arant KR, Loeser RF (2021) “Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review,” (in eng). JAMA 325(6):568–578. https://doi.org/10.1001/jama.2020.22171
Goldring MB, Goldring SR (2007) Osteoarthritis. J Cell Physiol 213(3):626–634. https://doi.org/10.1002/jcp.21258
Conaghan PG et al (2015) “Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies,” (in eng). Rheumatology (Oxford) 54(2):270–277. https://doi.org/10.1093/rheumatology/keu332
Gregori D et al (2018) Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA 320(24):2564–2579. https://doi.org/10.1001/jama.2018.19319
Lane NE et al (2011) OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 19(5):478–482. https://doi.org/10.1016/j.joca.2010.09.013
Van Spil WE, Kubassova O, Boesen M, Bay-Jensen AC, Mobasheri A (2019) “Osteoarthritis phenotypes and novel therapeutic targets,” (in eng). Biochem Pharmacol 165:41–48. https://doi.org/10.1016/j.bcp.2019.02.037
Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M (2016) “Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature,” (in eng). BMC Musculoskelet Disord 17(1):425. https://doi.org/10.1186/s12891-016-1286-2
Wang X et al (2017) Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthr Cartil 25(8):1304–1312. https://doi.org/10.1016/j.joca.2017.02.804
Mathiessen A, Conaghan PG (2017) “Synovitis in osteoarthritis: current understanding with therapeutic implications,” (in eng). Arthritis Res Ther 19(1):18. https://doi.org/10.1186/s13075-017-1229-9
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Pavelka K, Pavelková A, Olejárová M (2006) Methotrexate in the treatment of the erosive osteoarthrosis of the hands. Ann Rheum Dis 65(Suppl II):402
Ferrero S et al (2021) Methotrexate treatment in hand osteoarthritis refractory to usual treatments: A randomised, double-blind, placebo-controlled trial. Semin Arthritis Rheum 51(4):831–838. https://doi.org/10.1016/j.semarthrit.2021.04.016
Wang Y et al (2023) "Methotrexate to treat hand osteoarthritis with synovitis (METHODS): an Australian, multisite, parallel-group, double-blind, randomised, placebo-controlled trial," (in eng), Lancet https://doi.org/10.1016/s0140-6736(23)01572-6.
Enteshari-Moghaddam A, Isazadehfar K, Habibzadeh A, Hemmati M (2019) “Efficacy of Methotrexate on Pain Severity Reduction and Improvement of Quality of Life in Patients with Moderate to Severe Knee Osteoarthritis,” (in eng). Anesth Pain Med 9(3):e89990. https://doi.org/10.5812/aapm.89990
Holanda HTd, Pollak DF, Pucinelli MLC (2007) Low-dose methotrexate compared to placebo in the treatment of knee osteoarthritis. Revista Brasileira de Reumatologia 47(5):334–340. https://doi.org/10.1590/s0482-50042007000500008
Kingsbury SR et al (n.d.) "Significant pain reduction with oral methotrexate in knee osteoarthritis; results from the promote randomised controlled phase iii trial of treatment effectiveness."Elsevier.http://www.oarsijournal.com/article/S1063458419301621/abstract (accessed 19/5, 2023).
Wenham CY, Grainger AJ, Hensor EM, Caperon AR, Ash ZR, Conaghan PG (2013) Methotrexate for pain relief in knee osteoarthritis: an open-label study. Rheumatology (Oxford) 52(5):888–892. https://doi.org/10.1093/rheumatology/kes386
Zhu Z et al (2020) Can low-dose methotrexate reduce effusion-synovitis and symptoms in patients with mid- to late-stage knee osteoarthritis? Study protocol for a randomised, double-blind, and placebo-controlled trial. Trials 21(1):795. https://doi.org/10.1186/s13063-020-04687-3
Kvien TK (n.d.) "Methotrexate in Erosive Inflammatory Hand Osteoarthritis- ClinicalTrials.gov." https://clinicaltrials.gov/ct2/show/NCT04579848 (accessed).
Lee W et al (2018) “Efficacy of Hydroxychloroquine in Hand Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial,” (in eng). Arthritis Care Res (Hoboken) 70(9):1320–1325. https://doi.org/10.1002/acr.23471
Kedor C et al (2021) "Hydroxychloroquine in patients with inflammatory and erosive osteoarthritis of the hands: results of the OA-TREAT study-a randomised, double-blind, placebo-controlled, multicentre, investigator-initiated trial," (in eng), RMD Open 7(2) https://doi.org/10.1136/rmdopen-2021-001660.
Saviola G et al (2012) “Clodronate and hydroxychloroquine in erosive osteoarthritis: a 24-month open randomized pilot study,” (in eng). Mod Rheumatol 22(2):256–263. https://doi.org/10.1007/s10165-011-0506-8
Kingsbury SR et al (2018) “Hydroxychloroquine Effectiveness in Reducing Symptoms of Hand Osteoarthritis: A Randomized Trial,” (in eng). Ann Intern Med 168(6):385–395. https://doi.org/10.7326/m17-1430
Bonfante H, Machado L, Capp A, Paes M, Levy R, Teixeira HC (2008) Assessment of the use of hydroxychloroquine on knees’ osteoarthritis treatment. Revista Brasileira de Reumatologia 48:208–212
Jokar M, Mirfeizi Z, Keyvanpajouh K (2013) “The effect of hydroxychloroquine on symptoms of knee osteoarthritis: a double-blind randomized controlled clinical trial,” (in eng). Iran J Med Sci 38(3):221–226
Abou-Raya S, Abou-Raya A, Khadrawe T (2014) SAT0450 Efficacy of Hydroxychloroquine in the Treatment of Symptomatic Knee Osteoarthritis in Older Adults: A Randomized Placebo-Controlled Trial. Ann Rheum Dis 73:756–757. https://doi.org/10.1136/annrheumdis-2014-eular.2764
Fioravanti A, Fabbroni M, Cerase A, Galeazzi M (2009) “Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study,” (in eng). Rheumatol Int 29(8):961–965. https://doi.org/10.1007/s00296-009-0872-0
Chevalier X et al (2015) “Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: a randomised, multicentre, double-blind, placebo-controlled trial,” (in eng). Ann Rheum Dis 74(9):1697–1705. https://doi.org/10.1136/annrheumdis-2014-205348
Aitken D et al (2018) A randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis - the HUMOR trial. Osteoarthr Cartil 26(7):880–887. https://doi.org/10.1016/j.joca.2018.02.899
Kloppenburg M et al (2018) “Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial,” (in eng). Ann Rheum Dis 77(12):1757–1764. https://doi.org/10.1136/annrheumdis-2018-213202
Verbruggen G, Wittoek R, Vander Cruyssen B, Elewaut D (2012) “Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification,” (in eng). Ann Rheum Dis 71(6):891–8. https://doi.org/10.1136/ard.2011.149849
Wang J (2018) “Efficacy and safety of adalimumab by intra-articular injection for moderate to severe knee osteoarthritis: An open-label randomized controlled trial,” (in eng). J Int Med Res 46(1):326–334. https://doi.org/10.1177/0300060517723182
Cohen SB et al (2011) “A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee,” (in eng). Arthritis Res Ther 13(4):R125. https://doi.org/10.1186/ar3430
Chevalier X et al (2009) “Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study,” (in eng). Arthritis Rheum 61(3):344–352. https://doi.org/10.1002/art.24096
Fleischmann RM et al (2019) “A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis,” (in eng). Arthritis Rheumatol 71(7):1056–1069. https://doi.org/10.1002/art.40840
Wang SX et al (2017) “Safety, tolerability, and pharmacodynamics of an anti-interleukin-1α/β dual variable domain immunoglobulin in patients with osteoarthritis of the knee: a randomized phase 1 study,” (in eng). Osteoarthritis Cartilage 25(12):1952–1961. https://doi.org/10.1016/j.joca.2017.09.007
Cai G et al (2022) “Study protocol for a randomised controlled trial of diacerein versus placebo to treat knee osteoarthritis with effusion-synovitis (DICKENS),” (in English). Trials 23(1):768. https://doi.org/10.1186/s13063-022-06715-w
Schieker M et al (2020) “Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement : Exploratory Analyses From a Randomized, Double-Blind, Placebo-Controlled Trial,” (in eng). Ann Intern Med 173(7):509–515. https://doi.org/10.7326/m20-0527
Kloppenburg M et al (2019) “Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and anti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis,” (in eng). Ann Rheum Dis 78(3):413–420. https://doi.org/10.1136/annrheumdis-2018-213336
Gallelli L et al (2013) “The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis A randomized open label trial,” (in eng). Osteoarthritis Cartilage 21(9):1400–8. https://doi.org/10.1016/j.joca.2013.06.026
Gineyts E et al (2004) “Effects of ibuprofen on molecular markers of cartilage and synovium turnover in patients with knee osteoarthritis,” (in eng). Ann Rheum Dis 63(7):857–861. https://doi.org/10.1136/ard.2003.007302
Xu QX, Wittoek R (2020) “Influence of non-steroidal anti-inflammatory drugs on the inflammatory sonographic features in erosive hand osteoarthritis: an intervention study,” (in eng). Rheumatol Adv Pract 4(1):rkaa002. https://doi.org/10.1093/rap/rkaa002
Davis CR et al (2021) “Colchicine is not effective for reducing osteoarthritic hand pain compared to placebo: a randomised, placebo-controlled trial (COLAH),” (in eng). Osteoarthritis Cartilage 29(2):208–214. https://doi.org/10.1016/j.joca.2020.11.002
Døssing A et al (2023) “Colchicine twice a day for hand osteoarthritis (COLOR): a double-blind, randomised, placebo-controlled trial,” (in eng). Lancet Rheumatol 5(5):e254–e262. https://doi.org/10.1016/s2665-9913(23)00065-6
Das SK et al (2002) “A randomized controlled trial to evaluate the slow-acting symptom-modifying effects of colchicine in osteoarthritis of the knee: a preliminary report,” (in eng). Arthritis Rheum 47(3):280–284. https://doi.org/10.1002/art.10455
Das SK et al (2002) “A randomized controlled trial to evaluate the slow-acting symptom modifying effects of a regimen containing colchicine in a subset of patients with osteoarthritis of the knee,” (in eng). Osteoarthritis Cartilage 10(4):247–252. https://doi.org/10.1053/joca.2002.0516
Aran S, Malekzadeh S, Seifirad S (2011) “A double-blind randomized controlled trial appraising the symptom-modifying effects of colchicine on osteoarthritis of the knee,” (in eng). Clin Exp Rheumatol 29(3):513–8
Leung YY et al (2018) “Colchicine lack of effectiveness in symptom and inflammation modification in knee osteoarthritis (COLKOA): a randomized controlled trial,” (in eng). Osteoarthritis Cartilage 26(5):631–640. https://doi.org/10.1016/j.joca.2018.01.026
Kvien TK et al (2008) “Efficacy and safety of a novel synergistic drug candidate, CRx-102, in hand osteoarthritis,” (in eng). Ann Rheum Dis 67(7):942–948. https://doi.org/10.1136/ard.2007.074401
Wenham CY et al (2012) “A randomized, double-blind, placebo-controlled trial of low-dose oral prednisolone for treating painful hand osteoarthritis,” (in eng). Rheumatology (Oxford) 51(12):2286–2294. https://doi.org/10.1093/rheumatology/kes219
Kroon FPB et al (2019) “Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial,” (in eng). Lancet 394(10213):1993–2001. https://doi.org/10.1016/s0140-6736(19)32489-4
Keen HI, Wakefield RJ, Hensor EM, Emery P, Conaghan PG (2010) “Response of symptoms and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study,” (in eng). Rheumatology (Oxford) 49(6):1093–1100. https://doi.org/10.1093/rheumatology/keq010
Kuszewski JC, Wong RHX, Howe PRC (2020) “Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity,” (in eng). Rheumatol Adv Pract 4(2):rkaa036. https://doi.org/10.1093/rap/rkaa036
Hill CL et al (2016) Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis 75(1):23–29. https://doi.org/10.1136/annrheumdis-2014-207169
Deutsch L (2007) Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J Am Coll Nutr 26(1):39–48. https://doi.org/10.1080/07315724.2007.10719584
Suzuki Y, Fukushima M, Sakuraba K, Sawaki K, Sekigawa K (2016) Krill Oil Improves Mild Knee Joint Pain: A Randomized Control Trial. PLoS ONE 11(10):e0162769. https://doi.org/10.1371/journal.pone.0162769
Stonehouse W, Benassi-Evans B, Bednarz J, Vincent AD, Hall S, Hill CL (2022) “Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial,” (in eng). Am J Clin Nutr 116(3):672–685. https://doi.org/10.1093/ajcn/nqac125
Laslett LL et al (2024) "Krill Oil for Knee Osteoarthritis: A Randomized Clinical Trial," (in eng), Jama https://doi.org/10.1001/jama.2024.6063.
Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS (2016) “Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial,” (in eng). Inflammopharmacology 24(6):377–388. https://doi.org/10.1007/s10787-016-0289-9
Wang Z et al (2020) “Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion-Synovitis of Knee Osteoarthritis : A Randomized Trial,” (in English). Ann Intern Med 173(11):861–869. https://doi.org/10.7326/M20-0990
Zegels B, Crozes P, Uebelhart D, Bruyère O, Reginster JY (2013) “Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis Results of a randomized double blind placebo controlled study,” (in eng). Osteoarthritis Cartilage 21(1):22–7. https://doi.org/10.1016/j.joca.2012.09.017
Wildi LM et al (2011) Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double-blind, placebo-controlled pilot study using MRI. Ann Rheum Dis 70(6):982–989. https://doi.org/10.1136/ard.2010.140848
Rozendaal RM et al (2008) “Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial,” (in eng). Ann Intern Med 148(4):268–277. https://doi.org/10.7326/0003-4819-148-4-200802190-00005
McAlindon T, Formica M, LaValley M, Lehmer M, Kabbara K (2004) “Effectiveness of glucosamine for symptoms of knee osteoarthritis: results from an internet-based randomized double-blind controlled trial,” (in eng). Am J Med 117(9):643–649. https://doi.org/10.1016/j.amjmed.2004.06.023
Giordano N, Fioravanti A, Papakostas P, Montella A, Giorgi G, Nuti R (2009) “The efficacy and tolerability of glucosamine sulfate in the treatment of knee osteoarthritis: A randomized, double-blind, placebo-controlled trial,” (in eng). Curr Ther Res Clin Exp 70(3):185–196. https://doi.org/10.1016/j.curtheres.2009.05.004
Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G (2017) “Combined Treatment With Chondroitin Sulfate and Glucosamine Sulfate Shows No Superiority Over Placebo for Reduction of Joint Pain and Functional Impairment in Patients With Knee Osteoarthritis: A Six-Month Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial,” (in eng). Arthritis Rheumatol 69(1):77–85. https://doi.org/10.1002/art.39819
Clegg DO et al (2006) “Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis,” (in eng). N Engl J Med 354(8):795–808. https://doi.org/10.1056/NEJMoa052771
Fransen M et al (2015) Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis 74(5):851–858. https://doi.org/10.1136/annrheumdis-2013-203954
Yazici Y (2010) Long-term safety of methotrexate in the treatment of rheumatoid arthritis. Clin Exp Rheumato 28(5 Suppl 61):S65–S67
Cronstein BN, Aune TM (2020) “Methotrexate and its mechanisms of action in inflammatory arthritis,” (in eng). Nat Rev Rheumatol 16(3):145–154. https://doi.org/10.1038/s41584-020-0373-9
Sweeney SE, Corr M, Kimbler TB (2012) “Role of interferon regulatory factor 7 in serum-transfer arthritis: regulation of interferon-β production,” (in eng). Arthritis Rheum 64(4):1046–1056. https://doi.org/10.1002/art.33454
Kingsbury SR et al (2015) “Pain reduction with oral methotrexate in knee osteoarthritis, a pragmatic phase iii trial of treatment effectiveness (PROMOTE): study protocol for a randomized controlled trial,” (in eng). Trials 16:77. https://doi.org/10.1186/s13063-015-0602-8
Clark P et al (1993) “Hydroxychloroquine compared with placebo in rheumatoid arthritis A randomized controlled trial,” (in eng). Ann Intern Med 119(11):1067–71. https://doi.org/10.7326/0003-4819-119-11-199312010-00002
Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ (2000) “Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties,” (in eng). Blood 95(11):3460–3466
Vuolteenaho K, Kujala P, Moilanen T, Moilanen E (2005) “Aurothiomalate and hydroxychloroquine inhibit nitric oxide production in chondrocytes and in human osteoarthritic cartilage,” (in eng). Scand J Rheumatol 34(6):475–9. https://doi.org/10.1080/03009740510026797
Singh A, Kotlo A, Wang Z, Dissanayaka T, Das S, Antony B (2022) Efficacy and safety of hydroxychloroquine in osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Korean J Intern Med 37(1):210–221. https://doi.org/10.3904/kjim.2020.605
Pelletier JP, Martel-Pelletier J, Abramson SB (2001) “Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets,” (in eng). Arthritis Rheum 44(6):1237–1247. https://doi.org/10.1002/1529-0131(200106)44:6%3c1237::Aid-art214%3e3.0.Co;2-f
Kobayashi K et al (2000) “Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction,” (in eng). J Exp Med 191(2):275–286. https://doi.org/10.1084/jem.191.2.275
Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C (2013) “Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study,” (in eng). Ann Rheum Dis 72(4):535–540. https://doi.org/10.1136/annrheumdis-2011-201047
Arora T et al (2009) “Differences in binding and effector functions between classes of TNF antagonists,” (in eng). Cytokine 45(2):124–131. https://doi.org/10.1016/j.cyto.2008.11.008
Grunke M, Schulze-Koops H (2006) “Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade,” (in eng). Ann Rheum Dis 65(4):555–556. https://doi.org/10.1136/ard.2006.053272
Schett G, Dayer JM, Manger B (2016) “Interleukin-1 function and role in rheumatic disease,” (in eng). Nat Rev Rheumatol 12(1):14–24. https://doi.org/10.1038/nrrheum.2016.166
Pelletier JP et al (1997) “In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy,” (in eng). Arthritis Rheum 40(6):1012–1019. https://doi.org/10.1002/art.1780400604
Kamath RV et al (2012) Blockade of both il-1a and il-1b by a combination of monoclonal antibodies prevents the development and reverses established pain in a preclinical model of osteoarthritis. Osteoarthritis Cartilage 20:S62–S62
Chevalier X, Eymard F (2019) “Anti-IL-1 for the treatment of OA: dead or alive?,” (in eng). Nat Rev Rheumatol 15(4):191–192. https://doi.org/10.1038/s41584-019-0185-y
Fidelix TS, Macedo CR, Maxwell LJ, Fernandes Moça Trevisani V (2014) “Diacerein for osteoarthritis,” (in eng). Cochrane Database Syst Rev 2:Cd005117. https://doi.org/10.1002/14651858.CD005117.pub3
Malda J, Boere J, van de Lest CH, van Weeren P, Wauben MH (2016) “Extracellular vesicles — new tool for joint repair and regeneration,” (in eng). Nat Rev Rheumatol 12(4):243–249. https://doi.org/10.1038/nrrheum.2015.170
da Costa BR et al (2021) Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 375:n2321. https://doi.org/10.1136/bmj.n2321
Duong V, Oo WM, Ding C, Culvenor AG, Hunter DJ (2023) Evaluation and Treatment of Knee Pain: A Review. JAMA 330(16):1568–1580. https://doi.org/10.1001/jama.2023.19675
Conley B et al (2023) Core Recommendations for Osteoarthritis Care: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res (Hoboken) 75(9):1897–1907. https://doi.org/10.1002/acr.25101
Bindu S, Mazumder S, Bandyopadhyay U (2020) “Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective,” (in eng). Biochem Pharmacol 180:114147. https://doi.org/10.1016/j.bcp.2020.114147
Haartmans MJJ et al (2022) “Evaluation of the Anti-Inflammatory and Chondroprotective Effect of Celecoxib on Cartilage Ex Vivo and in a Rat Osteoarthritis Model,” (in eng). Cartilage 13(3):19476035221115540. https://doi.org/10.1177/19476035221115541
Aguiar GC et al (2016) “Mefenamic acid decreases inflammation but not joint lesions in experimental osteoarthritis,” (in eng). Int J Exp Pathol 97(6):438–446. https://doi.org/10.1111/iep.12216
Luitjens J et al (2023) Association Of Non-Steroidal Anti-Inflammatory Drugs On Synovitis And The Progression Of Osteoarthritis: Data From The Osteoarthritis Initiative. Osteoarthritis Cartilage 31:S411–S412
Ghlichloo I, Gerriets V (2024) "Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)," in StatPearls. Treasure Island (FL): StatPearls PublishingCopyright © 2024, StatPearls Publishing LLC.
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J (2006) “Gout-associated uric acid crystals activate the NALP3 inflammasome,” (in eng). Nature 440(7081):237–241. https://doi.org/10.1038/nature04516
Arroll B, Goodyear-Smith F (2004) “Corticosteroid injections for osteoarthritis of the knee: meta-analysis,” (in eng). Bmj 328(7444):869. https://doi.org/10.1136/bmj.38039.573970.7C
Creamer P (1997) “Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how?,” (in eng). Ann Rheum Dis 56(11):634–636. https://doi.org/10.1136/ard.56.11.634
Karim Z et al (2007) “Response to intramuscular methyl prednisolone in inflammatory hand pain: evidence for a targeted clinical, ultrasonographic and therapeutic approach,” (in eng). Ann Rheum Dis 66(5):690–692. https://doi.org/10.1136/ard.2006.061861
McAlindon TE et al (2017) Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 317(19):1967–1975. https://doi.org/10.1001/jama.2017.5283
Tou JC, Jaczynski J, Chen YC (2007) “Krill for human consumption: nutritional value and potential health benefits,” (in eng). Nutr Rev 65(2):63–77. https://doi.org/10.1111/j.1753-4887.2007.tb00283.x
Cleland LG, James MJ (2012) “Osteoarthritis. Omega-3 fatty acids and synovitis in osteoarthritic knees,” (in eng). Nat Rev Rheumatol 8(6):314–5. https://doi.org/10.1038/nrrheum.2012.60
James MJ, Cleland LG (1997) “Dietary n-3 fatty acids and therapy for rheumatoid arthritis,” (in eng). Semin Arthritis Rheum 27(2):85–97. https://doi.org/10.1016/s0049-0172(97)80009-1
Senftleber NK et al (2017) “Marine Oil Supplements for Arthritis Pain: A Systematic Review and Meta-Analysis of Randomized Trials,” (in eng). Nutrients 9(1):42. https://doi.org/10.3390/nu9010042
Ierna M, Kerr A, Scales H, Berge K, Griinari M (2010) “Supplementation of diet with krill oil protects against experimental rheumatoid arthritis,” (in eng). BMC Musculoskelet Disord 11:136. https://doi.org/10.1186/1471-2474-11-136
Goel A, Kunnumakkara AB, Aggarwal BB (2008) “Curcumin as ‘Curecumin’: from kitchen to clinic,” (in eng). Biochem Pharmacol 75(4):787–809. https://doi.org/10.1016/j.bcp.2007.08.016
Dai W, Yan W, Leng X, Chen J, Hu X, Ao Y (2021) “Effectiveness of Curcuma longa extract versus placebo for the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials,” (in eng). Phytother Res 35(11):5921–5935. https://doi.org/10.1002/ptr.7204
Ronca F, Palmieri L, Panicucci P, Ronca G (1998) “Anti-inflammatory activity of chondroitin sulfate,” (in eng). Osteoarthritis Cartilage 6(Suppl A):14–21. https://doi.org/10.1016/s1063-4584(98)80006-x
Omata T, Itokazu Y, Inoue N, Segawa Y (2000) “Effects of chondroitin sulfate-C on articular cartilage destruction in murine collagen-induced arthritis,” (in eng). Arzneimittelforschung 50(2):148–153. https://doi.org/10.1055/s-0031-1300180
Pelletier JP et al (2016) “Chondroitin sulfate efficacy versus celecoxib on knee osteoarthritis structural changes using magnetic resonance imaging: a 2-year multicentre exploratory study,” (in eng). Arthritis Res Ther 18(1):256. https://doi.org/10.1186/s13075-016-1149-0
Leeb BF, Schweitzer H, Montag K, Smolen JS (2000) “A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis,” (in eng). J Rheumatol 27(1):205–211
Hoffer LJ, Kaplan LN, Hamadeh MJ, Grigoriu AC, Baron M (2001) “Sulfate could mediate the therapeutic effect of glucosamine sulfate,” (in eng). Metabolism 50(7):767–770. https://doi.org/10.1053/meta.2001.24201
Zhu X, Sang L, Wu D, Rong J, Jiang L (2018) “Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials,” (in eng). J Orthop Surg Res 13(1):170. https://doi.org/10.1186/s13018-018-0871-5
Kolasinski SL et al (2020) “2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee,” (in eng). Arthritis Care Res (Hoboken) 72(2):149–162. https://doi.org/10.1002/acr.24131
Acknowledgements
Not applicable
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by the National Natural Science Foundation of China (82103933).
Author information
Authors and Affiliations
Contributions
Rui Zhu, Haonan Fang, Junjie Wang, Liru Ge, Xiaoyue Zhang, Dawn Aitken, Guoqi Cai have made substantial contributions to all of the following: 1) the conception and design of the study, 2) drafting the article or revising it critically for important intellectual content, 3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We confirm that this manuscript has not been published elsewhere and is also not under consideration by another journal. All authors have approved the manuscript and agree with the submission.
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui Zhu and Haonan Fang Joint first authors.
Dawn Aitken and Guoqi Cai Joint senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, R., Fang, H., Wang, J. et al. Inflammation as a therapeutic target for osteoarthritis: A literature review of clinical trials. Clin Rheumatol 43, 2417–2433 (2024). https://doi.org/10.1007/s10067-024-07042-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-07042-y