Abstract
Objectives
Gout is characterized by hyperuricemia and recurrent inflammatory episodes caused by intra-articular crystal deposition of monosodium urate (MSU). There is a clear relationship between gout and metabolic syndrome. Recent evidence indicates that perforin plays a role in regulating glucose homeostasis and provides protection in diet-induced non-alcoholic steatohepatitis models. However, the impact of perforin on immune inflammation in gout remains unclear.
Methods
We induced acute gout models in both wild-type (WT) mice and Prf1null mice by administering intra-articular injections of MSU crystals. We compared the ankle joint swelling and the histological score between the two groups. Furthermore, we investigated underlying mechanisms through in vitro co-culture experiments involving CD8 T cells and macrophages.
Results
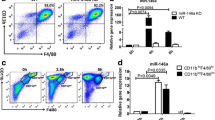
In this study, Prf1null mice showed significantly more pronounced ankle swelling with increased inflammatory cell infiltrations compared with WT mice 24 h after local MSU injection. Moreover, MSU-induced Prf1null mice exhibited increased accumulation of CD8 T cells but not NK cells. Perforin-deficient CD8 T cells displayed reduced cytotoxicity towards bone marrow–derived M0 and M1 macrophages and promoted TNF-α secretion from macrophage.
Conclusions
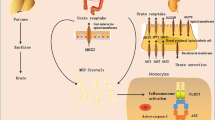
Perforin from CD8 T cells limits joint inflammation in mice with acute gout by downregulating macrophage-mediated inflammation.
Key Points • Perforin deficiency increased swelling in the ankle joints of mice upon MSU injection. • Perforin deficiency is associated with increased immune cell recruitment and severe joint damage in gout. • Perforin regulated CD8 T cell accumulation in gout and promoted CD8 T cell cytotoxicity towards M0 and M1 macrophages. • CD8 T cell-derived perforin regulated pro-inflammatory cytokine secretion of macrophage. |





Similar content being viewed by others
Data availability
Data are available upon reasonable request. Requests for data should be directed to the principal investigator, Dr. Yanying Liu (Liuyanying6850@126.com). Additionally, one study author has full access to all the data in the study and takes responsibility for its integrity and the data analysis.
References
Mikuls TR (2022) Gout. N Engl J Med 387(20):1877–87
Liu YR, Wang JQ, Li J (2023) Role of NLRP3 in the pathogenesis and treatment of gout arthritis. Front Immunol 14:1137822
Abhishek A, Roddy E, Doherty M (2017) Gout - a guide for the general and acute physicians. Clin Med (Lond) 17(1):54–59
Shi C, Zhou Z, Chi X, Xiu S, Yi C, Jiang Z, Chen R, Zhang L, Liu Z (2023) Recent advances in gout drugs. Eur J Med Chem 245(Pt 1):114890
Terkeltaub R (2017) What makes gouty inflammation so variable? BMC Med 15(1):158
Drivelegka P, Jacobsson LTH, Lindstrom U, Bengtsson K, Dehlin M (2023) Incident gout and risk of first-time acute coronary syndrome: a prospective, population-based cohort study in Sweden. Arthritis Care Res (Hoboken) 75(6):1292–1299
Oka P, Chong WM, Ng DX, Aau WK, Tan NC (2023) Epidemiology and risk factors associated with gout control among adult Asians: a real-world retrospective cohort study. Front Med (Lausanne) 10:1253839
Alduraibi FK, Saleem M, Ricart K, Patel RP, Szalai AJ, Singh JA (2023) Clustering patients with gout based on comorbidities and biomarkers: a cross-sectional study. J Rheumatol 50(6):817–826
Feldman N, Rotter-Maskowitz A, Okun E (2015) DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev 24(Pt A):29–39
Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, Shi SY, Schroer S, Luk CT, Lin GH, Mak TW, Woo M, Winer S, Winer DA (2015) Perforin is a novel immune regulator of obesity-related insulin resistance. Diabetes 64(1):90–103
Wang T, Sun G, Wang Y, Li S, Zhao X, Zhang C, Jin H, Tian D, Liu K, Shi W, Tian Y, Zhang D (2019) The immunoregulatory effects of CD8 T-cell-derived perforin on diet-induced nonalcoholic steatohepatitis. FASEB J 33(7):8490–8503
Queiroz-Junior CM, Madeira MF, Coelho FM, Costa VV, Bessoni RL, Sousa LF, Garlet GP, Souza Dda G, Teixeira MM, Silva TA (2011) Experimental arthritis triggers periodontal disease in mice: involvement of TNF-alpha and the oral microbiota. J Immunol 187(7):3821–3830
Wang B, Chen S, Qian H, Zheng Q, Chen R, Liu Y, Shi G (2020) Role of T cells in the pathogenesis and treatment of gout. Int Immunopharmacol 88:106877
Chen J, Wu M, Yang J, Wang J, Qiao Y, Li X (2017) The immunological basis in the pathogenesis of gout. Iran J Immunol 14(2):90–98
Badovinac VP, Hamilton SE, Harty JT (2003) Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18(4):463–474
Voskoboinik I, Whisstock JC, Trapani JA (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15(6):388–400
Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, Mazier D, Combadiere B (2003) Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol 170(4):2221–2228
Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF (2005) Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 52(1):201–211
Janka GE (2012) Familial and acquired hemophagocytic lymphohistiocytosis. Annu Rev Med 63:233–246
Zhong L, Li S, Wen Y, Zheng J, Liu F, Cao D, Liu Y (2020) Expansion of polymorphonuclear myeloid-derived suppressor cells in patients with gout. Front Immunol 11:567783
Alivernini S, MacDonald L, Elmesmari A, Finlay S, Tolusso B, Gigante MR, Petricca L, Di Mario C, Bui L, Perniola S, Attar M, Gessi M, Fedele AL, Chilaka S, Somma D, Sansom SN, Filer A, McSharry C, Millar NL, Kirschner K, Nerviani A, Lewis MJ, Pitzalis C, Clark AR, Ferraccioli G, Udalova I, Buckley CD, Gremese E, McInnes IB, Otto TD, Kurowska-Stolarska M (2020) Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat Med 26(8):1295–1306
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–364
Zhao J, Wei K, Jiang P, Chang C, Xu L, Xu L, Shi Y, Guo S, Xue Y, He D (2022) Inflammatory response to regulated cell death in gout and its functional implications. Front Immunol 13:888306
Amaral FA, Bastos LF, Oliveira TH, Dias AC, Oliveira VL, Tavares LD, Costa VV, Galvao I, Soriani FM, Szymkowski DE, Ryffel B, Souza DG, Teixeira MM (2016) Transmembrane TNF-alpha is sufficient for articular inflammation and hypernociception in a mouse model of gout. Eur J Immunol 46(1):204–211
Author information
Authors and Affiliations
Contributions
Yanying Liu and Tianqi Wang contributed to study conception and design. All authors contributed to experimental studies. Yanying Liu and Tianqi Wang contributed to analysis of data and drafting the manuscript. All authors had read and approved the final version to be published.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Zhang, C., Zhou, M. et al. CD8 T cell-derived perforin regulates macrophage-mediated inflammation in a murine model of gout. Clin Rheumatol (2024). https://doi.org/10.1007/s10067-024-06964-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10067-024-06964-x