Abstract
Objectives
To describe treatment patterns and persistence of tofacitinib, interleukin 17 inhibitors (IL-17Ai) and tumour necrosis factor inhibitors (TNFi), in patients with psoriatic arthritis (PsA).
Methods
Data from adult patients with PsA and who had received at least one prescription of tofacitinib, IL-17Ai or TNFi between May 2019 and September 2021 were sourced from the Australian OPAL dataset. Persistence, analysed via Kaplan–Meier methods, and propensity score matching between tofacitinib and bDMARD (IL-17Ai and TNFi) groups were conducted.
Results
Of 16,692 patients with PsA, 1486 (n = 406 tofacitinib, n = 416 IL-17Ai and n = 664 TNFi) were included. More females were in the tofacitinib group (75.4%) than in the IL-17Ai (61.1%) and TNFi (64.8%) groups. Overall, 19.2% of tofacitinib patients were first line, compared with 41.8% of IL-17Ai and 62.8% of TNFi patients. In the overall population, the median persistence was 16.5 months (95% CI 13.8 to 19.5 months), 17.7 months (95% CI 15.8 to 19.6 months) and 17.2 months (95% CI 14.9 to 20.5 months) in the tofacitinib, IL-17Ai and TNFi groups, respectively. Persistence was similar in the tofacitinib/IL-17Ai matched population; however, in the tofacitinib/TNFi matched population, persistence was longer in the tofacitinib group (18.7 months, 95% CI 15.6 to 21.4 months) compared with the TNFi group (12.2 months, 95% CI 19.9 to 14.9 months).
Conclusions
In this Australian real-world dataset, tofacitinib was more frequently used in later lines and among a slightly higher proportion of female patients than IL-17Ai or TNFi. Overall, treatment persistence was similar for tofacitinib, IL-17Ai and TNFi, but tofacitinib exhibited longer persistence than TNFi in a matched population.
Key Points • This is the first, large real-world study from Australia investigating the demographics, treatment patterns and comparative treatment persistence of patients with psoriatic arthritis (PsA) treated with tofacitinib and biologic disease-modifying drugs (bDMARDs). • The study suggests that tofacitinib is an effective intervention in PsA with at least comparable persistence to bDMARDs: tumour necrosis factor inhibitors (TNFi) and interleukin-17 A inhibitors (IL-17Ai). |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is an immune-mediated inflammatory condition associated with musculoskeletal manifestations and psoriasis (PsO). Global estimates of the prevalence of PsA vary from 0.3 to 1% of the population [1] and are typically reported to affect males and females equally between the ages of 40 and 50 years [2]. PsA is clinically heterogeneous with patients often presenting with a variety of symptoms including peripheral arthritis, axial disease, enthesitis, dactylitis, PsO, nail disease (pitting, onycholysis, hyperkeratosis, etc.), uveitis and inflammatory bowel disease (IBD) [3, 4]. Common comorbidities such as osteoporosis, diabetes and cardiovascular disease are also associated with increased mortality risk in patients with PsA [5, 6]. Although not fully elucidated, aberrant activation of the immune system and hyper-expression of pro-inflammatory cytokines are believed to play a key role in the pathogenesis of PsA [7].
The diversity of musculoskeletal manifestations and the presence of comorbidities can make the management of PsA complex. Fortunately, advances in therapies, namely the biological disease-modifying anti-rheumatic drugs (bDMARDs) and more recently, the newer class of targeted synthetic DMARDS (tsDMARDs), the Janus kinase inhibitors (JAKi), have provided potent tools with which to control many facets of the disease. The bDMARD therapies available for the treatment of PsA act to block some of the major pro-inflammatory cytokines implicated in the pathogenesis of this disease, namely tumour necrosis factor α (TNF α), interleukin 17A (IL-17A), interleukin 12/23 (IL-12/23) and interleukin 23 (IL-23) [8]. Janus kinases are important intracellular tyrosine kinases (JAK1, JAK2, JAK3 and Tyk2 (tyrosine kinase 2)) that also directly or indirectly regulate many of the cytokines involved in PsA. Tofacitinib was the first JAKi approved and reimbursed in Australia for the treatment of PsA in 2019 and in cellular settings preferentially inhibits JAK1 and JAK3, with lesser activity at JAK2 [9, 10]. Since then, upadacitinib has been approved for reimbursement but was not available for the treatment of PsA during the timeframe of this study.
While the efficacy and safety of tofacitinib at improving the signs and symptoms of active PsA were shown in the two global phase 3 studies; Oral Psoriatic Arthritis Trial (OPAL) Broaden and OPAL Beyond [11, 12], real-world data describing the characteristics and outcomes of patients who receive tofacitinib for the management of PsA is limited.
In Australia, the cost of b/tsDMARD therapy for the treatment of PsA is subsided by the Pharmaceutical Benefits Scheme (PBS) if a patient has severe PsA with demonstratable active disease.
OPAL Rheumatology is a consortium of Australian rheumatologists who use a bespoke electronic medical record (EMR) in their routine clinical practice. This study aimed to use the OPAL dataset to provide real-world evidence on the clinical effectiveness, treatment persistence and treatment patterns, for patients with PsA being treated with tofacitinib in the post-approval setting. Similar data were collected for patients treated with bDMARDs to provide context in a large real-world clinical practice setting.
Methods
Study design and setting
This was a retrospective cohort study describing the treatment persistence, clinical effectiveness and treatment patterns in patients with PsA prescribed tofacitinib or bDMARDs. At present 112 rheumatologists (approximately one-third of Australian rheumatologists) practising in 43 predominantly private clinics around Australia are contributing their clinical records to this initiative. De-identified clinical data captured at the point-of-care is extracted from all participating sites on a quarterly basis and aggregated to create the Optimising Patient outcomes in Australian rheumatoLogy (OPAL) dataset [13]. Clinical information captured during routine consultations was entered into patients’ EMR (Audit4, Software4Specialists Pty Ltd, Australia) by the clinician. Pathology reports were electronically transferred from pathology providers and deposited into the record. Information on the dataset has been published previously [13]. Diagnoses were made by individual rheumatologists rather than being criteria based. Data de-identified for patient, clinic and clinician were exported from each OPAL member’s local server, aggregated across all sites and analysed based on a predefined protocol.
The activities of OPAL Rheumatology Ltd have received overarching ethics approval from the University of New South Wales (UNSW) Human Research Ethics Committee (HREC), based on a patient opt-out arrangement (HC17799). This research protocol was approved by the UNSW HREC (HC200786).
Participants
Patients were included if they were registered in the OPAL dataset with a clinical diagnosis of PsA, were aged between 18 and 95 years of age and had received a prescription for a bDMARD or tsDMARD in the period 01 May 2019 to 30 September 2020. There was a minimum of 1-year follow-up for all sampled patients and therefore data up to 30 September 2021 were included in the study. Minimum follow-up in each group was 12 months (by design) and the median was 22 months in each group. The b/tsDMARDs that were approved for use in Australia and included in this study were adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, ixekizumab, secukinumab, tofacitinib and ustekinumab. Tofacitinib was the only tsDMARD included as no other JAKi were approved in Australia at the time of the study. Although patients on b/tsDMARDs other than tofacitinib, IL-17Ai or TNFi were planned to be included in the analyses there were only a few patients on ustekinumab, and according to OPALs Data Governance Policy, results from these patients were not included. Patients who had no visit data recorded or had missing start dates for tofacitinib/bDMARD treatment were also not included.
Statistical and analytical assessment
The primary exposure of interest was an initial prescription for tofacitinib or a bDMARD identified during the sample selection window. The date of the first prescription after the start of the sample selection window served as the study index date and the beginning of the post-index period. Patients who had received a prescription of tofacitinib during the sample selection window were considered part of the ‘tofacitinib group’. All other patients were considered to be in the bDMARD group. Patients who discontinued treatment were followed up for a minimum period of 1 year from their date of index.
Treatment persistence was defined as the time (in consecutive months) from treatment initiation until treatment discontinuation. Duration of treatment of the index b/tsDMARD was estimated using Kaplan–Meier methods. An exploratory and descriptive analysis of the most common reasons for discontinuation was performed.
Treatment pattern evaluation included the percentage of patients receiving monotherapy or conventional synthetic DMARD (csDMARD) combination therapy (methotrexate, hydroxychloroquine, sulfasalazine, leflunomide) at index and at 3-monthly intervals. The effectiveness of treatments was assessed using change from baseline (index date) to 18 months in Disease Activity Score-28 three indices including C-reactive protein (DAS28(3)-CRP). This outcome measure was chosen as there was more complete data collected in the medical record to calculate the simpler DAS28(3)-CRP compared to lower levels of data (including patient global assessment) required for calculation of DAS28(4)-CRP, or more disease-specific measures for psoriatic arthritis, such as Disease Activity in Psoriatic Arthritis (DAPSA) or Minimal Disease Activity (MDA). Patients with scores less than 2.6 were classified as being in remission, scores 2.6 to < 3.2 were classified as low disease activity, scores 3.2 to < 5.1 were classified as moderate disease activity and scores ≥ 5.1 were classified as high disease activity [14].
In this retrospective, non-interventional setting, the length of follow-up was variable and also some patients had missing values for some measures at some timepoints. Therefore, all summaries include the number of observations. As this is a descriptive study, no imputation of missing data was performed. In this observational, non-randomised setting, propensity score matching was performed to enhance the comparability of treatment groups however comparisons between groups should still be interpreted with caution (nominal p-values are provided to aid interpretation).
Propensity score matching
Matched analysis sets were constructed to address the observational nature of the data. Propensity score matching was planned between the tofacitinib and each bDMARD group. Propensity score matching increases the comparability of the observed baseline characteristics in patients treated with tofacitinib and bDMARDs. The propensity score is the conditional probability of receiving treatment (e.g. tofacitinib versus IL-17Ai or tofacitinib vs TNFi), which was estimated using logistic regression. Covariates included age group, sex, treatment combination at index and line of therapy (first vs second vs third or greater). Treatment combinations at index and age category were included using indicator variables. The baseline treatment combination covariates were methotrexate monotherapy, methotrexate in combination with other csDMARD(s), csDMARD(s) other than methotrexate and neither methotrexate nor other csDMARD(s) (bDMARD monotherapy).
A calliper width of 0.20 resulted in most tofacitinib patients (> 70%) having a match selected for both matched populations and hence this calliper width was not varied. The success of the matching was determined by examining the propensity score distribution (density plot) in both the original sample and the matched sample, and by comparing standardised difference (in means and proportions) between the matched groups. A difference above 10% (0.1) is generally considered indicative of substantial difference/bias in that covariate.
Study size
The study size was pragmatic, sampling all available data in the OPAL dataset.
Results
Participants
Of 219,812 patients in the OPAL dataset, 16,692 had a diagnosis of PsA and 1486 had been initiated with a b/tsDMARD during the sampling window. Of these, 406 received treatment with tofacitinib, 416 an IL-17Ai (286 with secukinumab and 130 with ixekizumab) and 664 a TNFi (Table 1). There were no missing data on age, combination status or line of therapy (Table 1). Patients had a minimum of 12-month follow-up with a median follow-up of 22 months in each group. The mean (SD) age of tofacitinib, IL-17Ai and TNFi-treated patients were 55.6 (12.6), 52.7 (12.7) and 50.3 (14.6) years, respectively. A slightly higher proportion of female patients was treated with tofacitinib (75.4%) compared with IL-17Ai (61.1%) and TNFi (64.8%). Overall, 19.2% of patients receiving tofacitinib were first line compared with 41.8% of IL-17Ai and 62.8% of TNFi-treated patients. The mean (SD) time from symptom onset to treatment initiation was longer for patients receiving tofacitinib (141.0 (107.9) months) and IL-17Ai (141.6 (106.9) months) compared to TNFi-treated patients (107.3 (97.2) months). At index, patients treated with IL-17Ai had higher physician and patient global assessment of skin visual analogue scale scores than those recorded for tofacitinib or TNFi (Table 1).
In the tofacitinib/IL-17Ai propensity score-matched population, there were 269 patients treated with tofacitinib and 269 treated with IL-17Ai. In the tofacitinib/TNFi propensity score-matched population, there were 256 treated with tofacitinib and 256 treated with TNFi. Patient demographics for the overall population are presented in Table 1. Propensity score matching was generally successful, although there were some characteristics that were associated with an inability to find good matches (see Table 2 and 3). Unmatched patients in the tofacitinib/IL-17Ai set were female, tended to be older and were less likely to be on b/tsDMARD monotherapy. Most unmatched patients were on their third or later line of therapy. For the tofacitinib/TNFi set, unmatched patients were also female and older.
Treatment patterns
At treatment initiation, approximately 25% of TNFi-treated patients and one-third of patients treated with tofacitinib and IL-17Ai were prescribed b/tsDMARD monotherapy. Another third received treatment in combination with both methotrexate and another csDMARD for TNFi and ~ 30% for tofacitinib and IL-17i (Table 4). By 12 months, approximately 1 in 5 patients had ceased treatment with their b/tsDMARD, almost one-quarter were receiving b/tsDMARD monotherapy, just under 20% were receiving methotrexate with a csDMARD, and 20% were receiving methotrexate alone (Table 4). See also Supplementary Fig. 1.
Treatment persistence
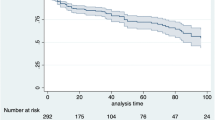
Treatment persistence was similar across all treatment groups (Fig. 1) in the unmatched population with a median persistence of 16.5 months (95% CI 13.8 to 19.5 months) in the tofacitinib group; 17.7 months (95% CI 15.8 to 19.6 months) in the IL-17Ai group and 17.2 months (95% CI 14.9 to 20.5 months) in the TNFi group (Fig. 1A). As might be expected, persistence in the first-line setting was longer: 22.0 months (95% CI 18.7 to not reached) in the tofacitinib group, 18.2 months (95% 16.2 to 22.6 months) in the IL-17Ai group and 26.1 months (95% CI 20.8 to not reached) in the TNFi group (Fig. 1B). In the tofacitinib/IL-17Ai matched population, median persistence was similar, 17.4 months (95% CI 13.8 to 20.3 months) in the tofacitinib group and 18.0 (95% CI 15.8 to 19.6 months) in the IL-17Ai group (Fig. 1C). In the tofacitinib/TNFi matched population, persistence was longer in the tofacitinib group (18.7 months, 95% CI 15.6 to 21.4 months) compared with the TNFi group (12.2 months, 95% CI 19.9 to 14.9 months; Fig. 1D).
Around half of tofacitinib (n = 139, 50.5%), IL-17Ai (n = 157 (53.4%) and TNFi (n = 241, 50.3%) patients ceased their index b/tsDMARD. The reason for discontinuing treatment was documented by the rheumatologist in the patient’s EMR at the time of the decision from a pre-defined menu. Lack of efficacy (including primary failure, secondary failure and partial response) was recorded in 28% of patients treated with tofacitinib, 28% of patients treated with an IL-17Ai and 31% of patients treated with TNFi. Adverse reaction was recorded in 15.1%, 7.0% and 6.2% of patients treated with tofacitinib, IL-17Ai and TNFi, respectively and ‘better alternative’ was given as a reason for ceasing in 11.5% of tofacitinib patients, 8.9% of IL-17Ai and 10.4% of TNFi-treated patients. (A full list of reasons for discontinuation is provided in supplementary Table 1.)
Treatment effectiveness
Treatment effectiveness was assessed using the DAS28(3)-CRP measure. This is a three-variable measure based on swollen and tender joint counts and CRP but lacks the patient global assessment that is used in the DAS28-CRP. At index, 51.5%, 48.2% and 34.8% of patients treated with tofacitinib, IL17Ai and TNFi (respectively) were in remission or LDA based on their DAS28(3)-CRP scores, which increased to approximately 80% of patients by 3 months and was maintained to 12 months (Fig. 2A). There appear to be some numerical differences in efficacy at 6 months between both tofacitinib and IL-17Ai, with 78% of tofacitinib patients and 84% of IL-17Ai patients in remission or LDA (Fig. 2B) and 78% of tofacitinib and 87% of TNFi patients (Fig. 2C) achieving remission or LDA at 6 months. However, this difference did not persist and may be related to there being less numbers of patients with data at the 6-month timepoint.
Discussion
Our study reports the treatment persistence and treatment patterns of a large cohort of patients with PsA treated with b/tsDMARDs in a real-world setting in Australia. Almost 1500 patients are included from the overall dataset. Patients receiving tofacitinib were predominantly in later lines of treatment compared to those on IL-17Ai and TNFi. Consequently, their median age was slightly higher, at 55 years compared to 52 and 50 years for IL-17Ai and TNFi, respectively. The utilisation of newer treatments in later lines of therapy is a common trend. Older and more established bDMARDs like TNFi are frequently employed as the first-line therapy in PsA, likely due to the accrued level of familiarity rheumatologists have with their efficacy, safety profile, as well as their potential short- and long-term adverse events. Furthermore, patients experiencing multiple treatment failures may have additional factors at play, such as the presence of conditions like fibromyalgia, a high body mass index, and comorbidities, which can complicate their management. However, we should note that our study did not delve into factors associated with challenging-to-treat conditions.
Treatment persistence was found to be similar across the different treatment types in an unmatched PsA population, with median persistence of approximately 17 months in all lines of therapy, with longer persistence found in the first-line setting. This is similar to that observed in Swedish [15], French [16] and Israeli [17] cohorts of patients with PsA. This contrasts with rheumatoid arthritis patients from the OPAL dataset in whom median treatment persistence was approximately double that of our PsA cohort [18]. Differences in persistence between disease states have been reported previously, although the reasons for observed differences are unknown [19]. In the analysis of the matched population receiving tofacitinib and IL-17Ai therapies, the median duration of persistence was found to be comparable between the two groups while in the matched population of tofacitinib and TNFi recipients, the tofacitinib group was found to have longer median persistence. Limited data exists in the literature comparing the persistence of matched tofacitinib and TNFi treatment groups, so validation of these results from other sources will be of interest.
In Australia, tofacitinib, IL-17Ai and TNFi are indicated for treatment of PsA alone or in combination with csDMARDs for patients who have had an inadequate response to two prior DMARD therapies. Around one-third of patients treated with a non-TNFi (tofacitinib or IL-17Ai) were treated as monotherapy, while slightly less, ~ 25% of TNFi-treated patients, were treated as monotherapy. Recently, the role of methotrexate in severe PsA has been questioned due to the scarcity of supporting data which has mainly come from small inconclusive trials [20, 21]. The SEAM-PsA study in 2019 demonstrated that etanercept exhibited superior efficacy to MTX and the addition of methotrexate to etanercept made no substantial impact on the overall effectiveness of etanercept monotherapy [22]. The European Alliance of Associations for Rheumatology (EULAR) recommendations for the pharmacological treatment of PsA however continue to place MTX and other csDMARDs at the top of the treatment algorithm [23]. Although limited data comparing tofacitinib monotherapy vs combination therapy exist, a recent real-world study from the United States of America reported that persistence rates for monotherapy and combination tofacitinib therapy in PsA were similar after 6 months of treatment [24]. Furthermore, side effects from methotrexate such as fatigue commonly lead to discontinuation [25] and thus in practice treatment with b/tsDMARD monotherapy is common, as was observed in our study.
From the data that were available, using DAS28-3(CRP) as a surrogate for disease activity, on average, most patients had moderate to high disease activity at the time of index. All three treatments (tofacitinib, IL17Ai and TNFi) in the overall population and in the tofacitinib/IL-17Ai and tofacitinib/TNFi matched populations effectively increased the proportion of patients in remission or LDA to around 80% by 3 months of treatment, which was maintained out to 12 months.
Although persistence is a multifaceted measure that considers factors beyond treatment efficacy, including safety, tolerability and patient satisfaction or preferences, it is also often used as a surrogate for efficacy in the real world [26,27,28]. Using persistence as a broad marker of efficacy here, there were no differences between the 3 treatment groups. This is consistent with the findings of a network meta-analysis which found similar clinical efficacy for tofacitinib when compared with bDMARDs [29]. In this study, we used propensity scoring to match treated populations and found that the persistence on tofacitinib was greater than that on TNFi but not IL-17Ai. We are not aware of other studies that have directly compared persistence on tofacitinib with bDMARDs using this approach.
Limitations
There are several limitations to our study. Given the nature of data collection in this study, missing data are inevitable, and thus some assessments were hampered by missing data. This led to the use of a non-disease specific measure, the DAS28(3)-CRP, being used as an outcome measure for disease activity. While the DAS28 is commonly used to measure disease activity in PsA patients, it was not originally designed for this purpose. This study was unable to use a more PsA-specific measure, such as the DAPSA, due to limitations in real-world clinical practice. In Australia, the routine collection of DAPSA scores and skin assessments is not widespread, and only a subset of rheumatologists incorporates this measure in their regular clinical evaluation of patients. These findings underscore the challenges associated with using more specialised measures in routine clinical care and highlight some of the limitations of real-world studies.
The direction of missing data bias depends on the measure being assessed. It might be reasonable to expect, for example, that those with troublesome skin manifestations are more likely to have a physician or patient global assessment of skin performed. The sample size, variables and study duration were selected to minimise the impact of this. As suggested previously [18], we assume, however, given the source of this data, that data are likely to be missing at random, as important clinical information is likely to have been reported within the patient’s medical record. The results from the matched analyses may not be generalisable to the patient population as there were a substantial proportion (around one-third) of tofacitinib patients for whom good matches on other treatments could not be found. In addition, not all variables that could potentially influence choice of prescription were collected and hence could not be included in the propensity score model (e.g. dactylitis, enthesitis, severe skin involvement, axial involvement, involvement of lung, cardiovascular, skin). This may lead to biased estimates of differences between groups.
Conclusions
In this analysis of a large real-world dataset in Australia, tofacitinib was seen to be more commonly utilised in later lines of therapy and in a slightly higher proportion of female patients than IL-17Ai or TNFi. Treatment persistence was similar for tofacitinib, IL-17Ai and TNFi in the overall population, with longer persistence than TNFi in a matched population.
Data availability
Data collection is based on opt-out patient consent, and patients have consented to their data being made available to OPAL Rheumatology only. Requests for access to summary statistics will be considered by the OPAL Scientific Review Committee.
References
Gladman DD, Antoni C, Mease P, Clegg DO, Nash P (2005) Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 64(Suppl 2):ii14-7. https://doi.org/10.1136/ard.2004.032482
Husni ME, Betts KA, Griffith J, Song Y, Ganguli A (2017) Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int 37(9):1423–1434. https://doi.org/10.1007/s00296-017-3760-z
Bolt JW, van Ansenwoude CMJ, Hammoura I, van de Sande MG, van Baarsen LGM (2021) Translational research studies unraveling the origins of psoriatic arthritis: moving beyond skin and joints. Front Med (Lausanne) 8:711823. https://doi.org/10.3389/fmed.2021.711823
Ocampo DV, Gladman D (2019) Psoriatic arthritis. F1000Res 8. https://doi.org/10.12688/f1000research.19144.1
Sukhov A, Adamopoulos IE, Maverakis E (2016) Interactions of the immune system with skin and bone tissue in psoriatic arthritis: a comprehensive review. Clin Rev Allergy Immunol 51(1):87–99. https://doi.org/10.1007/s12016-016-8529-8
Berekmeri A, Mahmood F, Wittmann M, Helliwell P (2018) Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev Clin Immunol 14(9):719–730. https://doi.org/10.1080/1744666X.2018.1512404
Crispino N, Ciccia F (2021) JAK/STAT pathway and nociceptive cytokine signalling in rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol 393:668–75
Carvalho AL, Hedrich CM (2021) The molecular pathophysiology of psoriatic arthritis-the complex interplay between genetic predisposition, epigenetics factors, and the microbiome. Front Mol Biosci 8:662047. https://doi.org/10.3389/fmolb.2021.662047
Chen M, Dai SM (2020) A novel treatment for psoriatic arthritis: Janus kinase inhibitors. Chin Med J (Engl) 133(8):959–967. https://doi.org/10.1097/CM9.0000000000000711
Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, Chan GL, Krishnaswami S, Prakash C (2014) The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab Dispos 42(4):759–773. https://doi.org/10.1124/dmd.113.054940
Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, Kudlacz E, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 377(16):1525–1536. https://doi.org/10.1056/NEJMoa1615977
Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, Cieslak D, Graham D, Wang C, Menon S, Hendrikx T, Kanik KS (2017) Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 377(16):1537–1550. https://doi.org/10.1056/NEJMoa1615975
Littlejohn GO, Tymms KE, Smith T, Griffiths HT (2020) Using big data from real-world Australian rheumatology encounters to enhance clinical care and research. Clin Exp Rheumatol 38(5):874–880
Madsen OR (2013) Agreement between the DAS28-CRP assessed with 3 and 4 variables in patients with rheumatoid arthritis treated with biological agents in the daily clinic. J Rheumatol 40(4):379–385. https://doi.org/10.3899/jrheum.120594
Geale K, Lindberg I, Paulsson EC, Wennerstrom ECM, Tjarnlund A, Noel W, Enkusson D, Theander E (2020) Persistence of biologic treatments in psoriatic arthritis: a population-based study in Sweden. Rheumatol Adv Pract 4(2):rkaa070. https://doi.org/10.1093/rap/rkaa070
Pina Vegas L, Penso L, Claudepierre P, Sbidian E (2022) Long-term Persistence of first-line biologics for patients with psoriasis and psoriatic arthritis in the french health insurance database. JAMA Dermatol 158(5):513–522. https://doi.org/10.1001/jamadermatol.2022.0364
Haddad A, Saliba W, Lavi I, Batheesh A, Kasem S, Gazitt T, Feldhamer I, Cohen AD, Zisman D (2022) The Association of psoriatic arthritis with all-cause mortality and leading causes of death in psoriatic arthritis. J Rheumatol 49(2):165–170. https://doi.org/10.3899/jrheum.210159
Bird P, Littlejohn G, Butcher B, Smith T, da Fonseca PC, Witcombe D, Griffiths H (2020) Real-world evaluation of effectiveness, persistence, and usage patterns of tofacitinib in treatment of rheumatoid arthritis in Australia. Clin Rheumatol 39(9):2545–2551. https://doi.org/10.1007/s10067-020-05021-7
Murage MJ, Tongbram V, Feldman SR, Malatestinic WN, Larmore CJ, Muram TM, Burge RT, Bay C, Johnson N, Clifford S, Araujo AB (2018) Medication adherence and persistence in patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis: a systematic literature review. Patient Prefer Adherence 12:1483–1503. https://doi.org/10.2147/PPA.S167508
Felten R, Lambert De Cursay G, Lespessailles E (2022) Is there still a place for methotrexate in severe psoriatic arthritis? Ther Adv Musculoskelet Dis 14:1759720X221092376. https://doi.org/10.1177/1759720X221092376
Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, Mulherin DM, Kitas GD, Chakravarty K, Tom BD, O’Keeffe AG, Maddison PJ, Scott DL (2012) A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 51(8):1368–1377. https://doi.org/10.1093/rheumatology/kes001
Mease PJ, Gladman DD, Collier DH, Ritchlin CT, Helliwell PS, Liu L, Kricorian G, Chung JB (2019) Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase III trial. Arthritis Rheumatol 71(7):1112–1124. https://doi.org/10.1002/art.40851
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewe RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79(6):700–712. https://doi.org/10.1136/annrheumdis-2020-217159
Mease PJ, Young P, Gruben D, Fallon L, Germino R, Kavanaugh A (2022) Early real-world experience of tofacitinib for psoriatic arthritis: data from a United States healthcare claims database. Adv Ther 39(6):2932–2945. https://doi.org/10.1007/s12325-022-02084-7
Nowell WB, Karis E, Gavigan K, Stradford L, Zhao H, Chen L, Stryker S, Yun H, Venkatachalam S, Kricorian G, Xie F, Curtis JR (2022) Patient-reported nausea and fatigue related to methotrexate: a prospective, self-controlled study in the arthritispower((R)) registry. Rheumatol Ther 9(1):207–221. https://doi.org/10.1007/s40744-021-00398-6
Fagerli KM, Kearsley-Fleet L, Watson KD, Packham J, Contributors Group BR, Symmons DPM, Hyrich KL (2018) Long-term persistence of TNF-inhibitor treatment in patients with psoriatic arthritis. Data from the British Society for Rheumatology Biologics Register. RMD Open 4(1):e000596. https://doi.org/10.1136/rmdopen-2017-000596
Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, Askling J, Group AS (2015) Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 74(2):354–60. https://doi.org/10.1136/annrheumdis-2013-204128
Park EY, Lee SG, Park EK, Koo DW, Park JH, Kim GT, Tag HS, Kim HO, Suh YS (2018) Drug survival and the associated predictors in South Korean patients with rheumatoid arthritis receiving tacrolimus. Korean J Intern Med 33(1):193–202. https://doi.org/10.3904/kjim.2015.385
Gladman DD, Orbai AM, Gomez-Reino J, Chang-Douglass S, Leoncini E, Burton HE, Kanik KS, Romero AB, Cappelleri JC, Hsu MA (2020) Network meta-analysis of tofacitinib, biologic disease-modifying antirheumatic drugs, and apremilast for the treatment of psoriatic arthritis. Curr Ther Res Clin Exp 93:100601. https://doi.org/10.1016/j.curtheres.2020.100601
Acknowledgements
The authors would like to thank the members of OPAL Rheumatology Ltd and their patients for participating in this study, and Software4Specialists Pty Ltd for providing the Audit4 platform and de-identifying and aggregating the clinical data.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was sponsored by Pfizer. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Pfizer. Only employees of OPAL Rheumatology had access to study data and all analyses were completed by employees of OPAL Rheumatology.
Author information
Authors and Affiliations
Contributions
GL, JL, BB, MF, TS, COS, DW, HN and PY were involved in the design of the study. JL analysed the data. COS and BB wrote the first draft of the manuscript. All authors were involved in interpreting the results of the study, revising the manuscript and approving it prior to submission. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
The activities of OPAL Rheumatology Ltd have received overarching ethics approval from the University of New South Wales (UNSW) Human Research Ethics Committee (HREC), based on a patient opt-out arrangement (HC17799).
Consent to participate
This research protocol was approved by the UNSW HREC (HC200786). Opt-out arrangement for consent for research involving non-reidentifiable routine electronic medical records.
Consent for publication
Patients have consented to the publication of data on an opt-out basis. All data are de-identified.
Competing interests
COS and TS are employees of OPAL Rheumatology Ltd and declare no competing interests. JL and BB are independent statistical consultants, funded by OPAL. The following interests are declared for GL; Astra Zeneca, MF; AbbVie, Janssen, UCB, Pfizer and Lilly and PY; Abbvie, Eli Lilly, Celltrion, Pfizer, Sandoz. DW and HN are employees of Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Littlejohn, G., Leadbetter, J., Butcher, B.E. et al. Real-world evaluation of persistence, effectiveness and usage patterns of tofacitinib in treatment of psoriatic arthritis in Australia. Clin Rheumatol 43, 1579–1589 (2024). https://doi.org/10.1007/s10067-024-06930-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-024-06930-7